A review of bronchoscopy specimens indicates an unusual number of Mycobacterium fortuitum–positive cultures. Which of the following observations would be the MOST likely cause of this finding?
Bronchoscopes cleaned with sporicidal solution
Inadequate cleaning prior to disinfection
Rinsing with tap water
Drying with air or alcohol
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies nontuberculous mycobacteria (NTM), including Mycobacterium fortuitum, as organisms commonly associated with water sources, particularly potable water systems. An unusual increase in M. fortuitum–positive bronchoscopy cultures is most often linked to waterborne contamination during endoscope reprocessing, making rinsing with tap water the most likely cause.
Tap water is not sterile and may harbor NTM, which are resistant to standard municipal water treatment and capable of forming biofilms within plumbing systems. If bronchoscopes are rinsed with tap water after high-level disinfection and not followed by appropriate sterile or filtered water rinses and thorough drying, organisms such as M. fortuitum may contaminate internal channels. This can lead to pseudo-outbreaks, where cultures are positive due to contamination rather than true patient infection.
Option B, inadequate cleaning prior to disinfection, can contribute to overall reprocessing failure but is less specifically associated with NTM contamination patterns. Option A is unlikely, as sporicidal solutions are effective disinfectants. Option D, drying with air or alcohol, is a recommended step to reduce microbial growth and would not cause contamination.
For CIC® exam preparation, recognizing that tap water exposure during endoscope reprocessing is a classic source of nontuberculous mycobacteria contamination is a key concept in outbreak investigation and device reprocessing surveillance.
How can infection prevention and control programs BEST implement recommendations across different departments?
Provide targeted, understandable education to staff.
Use a generic policy for all areas without customization.
Avoid department-specific training to reduce redundancy.
Rely on senior leadership to enforce policies without input from staff.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that successful implementation of infection prevention recommendations depends on effective communication, engagement, and education tailored to the audience. Healthcare departments differ significantly in workflow, patient population, risk profile, and daily practices. Therefore, providing targeted, understandable education to staff is the most effective strategy to ensure recommendations are adopted and sustained.
Option A reflects best practice by aligning infection prevention guidance with the specific roles and responsibilities of staff in each department. Education that uses relevant examples, scenarios, and language improves comprehension, promotes buy-in, and supports behavior change. The Study Guide highlights that adult learners benefit most from education that is practical, interactive, and clearly applicable to their work environment.
Options B and C are ineffective because generic or non-customized approaches often fail to address department-specific challenges and may lead to confusion or poor compliance. Avoiding department-specific training ignores variations in risk and undermines accountability. Option D relies solely on enforcement rather than collaboration, which can result in resistance and decreased adherence.
For the CIC® exam, this question reinforces that infection prevention programs function best when they act as educators and partners, not just policy enforcers. Tailored education empowers staff, enhances compliance, and ultimately improves patient safety outcomes across diverse healthcare settings.
A healthcare worker experiences a percutaneous exposure to a patient with untreated HIV. The next step is to:
Initiate HIV post-exposure prophylaxis (PEP) within 2 hours.
Wait for HIV test results before starting treatment.
Offer post-exposure prophylaxis only if symptoms develop.
Retest for HIV after 6 months before deciding on PEP.
HIV post-exposure prophylaxis (PEP) should be initiated within 2 hours to be most effective.
Waiting for results (B) delays critical treatment.
PEP should always be offered after high-risk exposure, not only if symptoms develop (C).
Retesting after 6 months (D) is recommended but should not delay PEP initiation.
CBIC Infection Control References:
APIC Text, "Bloodborne Pathogens and PEP," Chapter 11.
An immunocompetent patient is diagnosed with active tuberculosis (TB). Which of the following sites of the disease is MOST likely to result in transmission to healthcare personnel?
Renal TB
Miliary TB
Laryngeal TB
Tuberculous meningitis
Laryngeal tuberculosis (TB) is highly contagious because it involves the upper respiratory tract, leading to direct aerosolized transmission of Mycobacterium tuberculosis through talking, coughing, or sneezing.
Why the Other Options Are Incorrect?
A. Renal TB – Genitourinary TB is not typically transmissible via airborne droplets.
B. Miliary TB – While systemic, it does not involve direct respiratory transmission.
D. Tuberculous meningitis – TB in the central nervous system is not spread through respiratory secretions.
CBIC Infection Control Reference
APIC confirms that laryngeal TB is one of the most infectious forms and requires Airborne Precautions
The infection preventionist (IP) is working with the Product Evaluation Committee to select a sporicidal disinfectant for Clostridioides difficile. An effective disinfectant for the IP to recommend is
quaternary ammonium compound.
phenolic.
isopropyl alcohol.
sodium hypochlorite.
The correct answer is D, "sodium hypochlorite," as it is an effective sporicidal disinfectant for Clostridioides difficile that the infection preventionist (IP) should recommend. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, Clostridioides difficile (C. difficile) is a spore-forming bacterium responsible for significant healthcare-associated infections (HAIs), and its spores are highly resistant to many common disinfectants. Sodium hypochlorite (bleach) is recognized by the Centers for Disease Control and Prevention (CDC) and the Environmental Protection Agency (EPA) as a sporicidal agent capable of inactivating C. difficile spores when used at appropriate concentrations (e.g., 1:10 dilution of household bleach) and with the recommended contact time (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). This makes it a preferred choice for environmental disinfection in outbreak settings or areas with known C. difficile contamination.
Option A (quaternary ammonium compound) is effective against many bacteria and viruses but lacks sufficient sporicidal activity against C. difficile spores, rendering it inadequate for this purpose. Option B (phenolic) has broad-spectrum antimicrobial properties but is not reliably sporicidal and is less effective against C. difficile spores compared to sodium hypochlorite. Option C (isopropyl alcohol) is useful for disinfecting surfaces and killing some pathogens, but it is not sporicidal and evaporates quickly, making it ineffective against C. difficile spores.
The IP’s recommendation of sodium hypochlorite aligns with CBIC’s emphasis on selecting disinfectants based on their efficacy against specific pathogens and adherence to evidence-based guidelines (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). Proper use, including correct dilution and contact time, is critical to ensure effectiveness, and the IP should collaborate with the Product Evaluation Committee to ensure implementation aligns with safety and regulatory standards (CDC Guidelines for Environmental Infection Control in Healthcare Facilities, 2019).
An infection preventionist has been asked to consult on disinfectant products for use in a long term care home. What should their primary concern be?
Patient care items are cleaned whenever visibly soiled.
An appropriate disinfectant should be available whenever items are used on patients known to be colonized with multi drug resistant organisms.
Disinfectant products should be compatible with the patient care devices used by the facility.
Disinfectant products should have a mild odor to reduce allergy concerns.
The most critical factor in choosing disinfectants in long-term care is compatibility with medical devices to prevent damage and ensure safety. Improper selection can compromise disinfection efficacy and equipment longevity.
The APIC/JCR Workbook highlights:
“Organizations should evaluate compatibility of disinfectant products with the materials used in patient care equipment. Incompatibility can lead to equipment degradation or malfunction”.
This ensures compliance with manufacturer instructions and preserves warranty and functionality.
An infection preventionist (IP) observes an increase in primary bloodstream infections in patients admitted through the Emergency Department. Poor technique is suspected when peripheral intravenous (IV) catheters are inserted. The IP should FIRST stratify infections by:
Location of IV insertion: pre-hospital, Emergency Department, or in-patient unit.
Type of dressing used: gauze, CHG impregnated sponge, or transparent.
Site of insertion: hand, forearm, or antecubital fossa.
Type of skin preparation used for the IV site: alcohol, CHG/alcohol, or iodophor.
When an infection preventionist (IP) identifies an increase in primary bloodstream infections (BSIs) associated with peripheral intravenous (IV) catheter insertion, the initial step in outbreak investigation and process improvement is to stratify the data to identify potential sources or patterns of infection. According to the Certification Board of Infection Control and Epidemiology (CBIC), the "Surveillance and Epidemiologic Investigation" domain emphasizes the importance of systematically analyzing data to pinpoint contributing factors, such as location, technique, or equipment use, in healthcare-associated infections (HAIs). The question specifies poor technique as a suspected cause, and the first step should focus on contextual factors that could influence technique variability.
Option A, stratifying infections by the location of IV insertion (pre-hospital, Emergency Department, or in-patient unit), is the most logical first step. Different settings may involve varying levels of training, staffing, time pressure, or adherence to aseptic technique, all of which can impact infection rates. For example, pre-hospital settings (e.g., ambulance services) may have less controlled environments or less experienced personnel compared to in-patient units, potentially leading to technique inconsistencies. The CDC’s Guidelines for the Prevention of Intravascular Catheter-Related Infections (2017) recommend evaluating the context of catheter insertion as a critical initial step in investigating BSIs, making this a priority for the IP to identify where the issue is most prevalent.
Option B, stratifying by the type of dressing used (gauze, CHG impregnated sponge, or transparent), is important but should follow initial location-based analysis. Dressings play a role in maintaining catheter site integrity and preventing infection, but their impact is secondary to the insertion technique itself. Option C, stratifying by the site of insertion (hand, forearm, or antecubital fossa), is also relevant, as anatomical sites differ in infection risk (e.g., the hand may be more prone to contamination), but this is a more specific factor to explore after broader contextual data is assessed. Option D, stratifying by the type of skin preparation used (alcohol, CHG/alcohol, or iodophor), addresses antiseptic efficacy, which is a key component of technique. However, without first understanding where the insertions occur, it’s premature to focus on skin preparation alone, as technique issues may stem from systemic factors across locations.
The CBIC Practice Analysis (2022) supports a stepwise approach to HAI investigation, starting with broad stratification (e.g., by location) to guide subsequent detailed analysis (e.g., technique-specific factors). This aligns with the CDC’s hierarchical approach to infection prevention, where contextual data collection precedes granular process evaluation. Therefore, the IP should first stratify by location to establish a baseline for further investigation.
Which of the following is the BEST study design for assessing the benefit of a new treatment?
Interrupted time series
Correlational study
Parallel group study
Randomized controlled trial
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies the randomized controlled trial (RCT) as the gold standard study design for assessing the benefit of a new treatment. RCTs are specifically designed to determine causality by minimizing bias and confounding variables through random assignment of participants to intervention and control groups. This ensures that differences in outcomes can be attributed with the highest level of confidence to the treatment being studied rather than to external factors.
In an RCT, participants are randomly allocated to receive either the new treatment or a comparison intervention (such as standard therapy or placebo). Randomization balances known and unknown risk factors between groups, while controlled conditions allow precise measurement of treatment effects. This design is particularly important when evaluating new therapies, medications, or interventions where efficacy and safety must be clearly demonstrated.
The other study designs listed are less rigorous for assessing treatment benefit. An interrupted time series is useful for evaluating system-level interventions over time but is more susceptible to confounding influences. A correlational study can identify associations but cannot establish cause and effect. A parallel group study without randomization lacks adequate control for bias and confounding.
For CIC® exam preparation, it is essential to recognize that when the objective is to assess the benefit or effectiveness of a new treatment, a randomized controlled trial provides the strongest and most reliable evidence, making it the best answer.
An outbreak of Candida auris is suspected in the infection preventionist's (IP) facility. The IP's investigation must be conducted in a standard method and communication is critical. Which first step is MOST important?
Conduct environmental cultures
Plan to prevent future outbreaks
Notify facility administration
Perform analytical studies
In an outbreak investigation, the first critical step is to notify facility administration and other key stakeholders. This ensures the rapid mobilization of resources, coordination with infection control teams, and compliance with regulatory reporting requirements.
Why the Other Options Are Incorrect?
A. Conduct environmental cultures – While environmental sampling may be necessary, it is not the first step. The outbreak must first be confirmed and administration alerted.
B. Plan to prevent future outbreaks – Prevention planning happens later after the outbreak has been investigated and controlled.
D. Perform analytical studies – Data analysis occurs after case definition and initial response measures are in place.
CBIC Infection Control Reference
APIC guidelines state that the first step in an outbreak investigation is confirming the outbreak and notifying key stakeholders.
The infection preventionist (IP) is reviewing a laboratory report that indicates the presence of Enterococcus faecium in a 76-year-old patient’s urine culture. The patient has no symptoms of a urinary tract infection. The IP’s accurate interpretation of this result is that the patient:
Should be placed in isolation due to the risk of airborne transmission.
Has an active infection and requires immediate treatment with antibiotics.
Is colonized with the bacteria and does not require treatment at this time.
Has a pseudo-infection, which could be caused by contamination of the sample.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes the importance of distinguishing colonization from infection when interpreting microbiology results. Colonization refers to the presence of microorganisms on or within the body without causing clinical signs or symptoms of disease. In older adults, especially those in healthcare settings, asymptomatic bacteriuria is common and does not meet criteria for a urinary tract infection (UTI).
In this scenario, the presence of Enterococcus faecium in a urine culture in the absence of urinary symptoms—such as dysuria, urgency, fever, or suprapubic pain—indicates colonization rather than infection. The Study Guide notes that treating asymptomatic bacteriuria does not improve patient outcomes and may contribute to antimicrobial resistance, adverse drug events, and unnecessary healthcare costs. Therefore, antibiotics are not indicated.
Option A is incorrect because Enterococcus species are not transmitted via the airborne route; Standard Precautions are sufficient. Option B is incorrect because laboratory findings alone do not define infection without corresponding clinical symptoms. Option D is less accurate because contamination is more likely with mixed flora or improper collection; isolation of a known urinary colonizer in an asymptomatic patient is more consistent with colonization.
Accurate interpretation of such findings supports antimicrobial stewardship principles and aligns with evidence-based infection prevention practices tested on the CIC® exam.
==========
When developing an exposure control plan, the MOST important aspect in the prevention of exposure to tuberculosis is:
Placement of the patient in an airborne infection isolation room.
Identification of a potentially infectious patient.
Prompt initiation of chemotherapeutic agents.
Use of personal protective equipment.
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is an airborne disease that poses a significant risk in healthcare settings, particularly through exposure to infectious droplets. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the "Prevention and Control of Infectious Diseases" domain, which includes developing exposure control plans, aligning with the Centers for Disease Control and Prevention (CDC) "Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Healthcare Settings" (2005). The question seeks the most important aspect of an exposure control plan to prevent TB exposure, requiring a prioritization of preventive strategies.
Option B, "Identification of a potentially infectious patient," is the most important aspect. Early identification of individuals with suspected or confirmed TB (e.g., through symptom screening like persistent cough, fever, or weight loss, or diagnostic tests like chest X-rays and sputum smears) allows for timely isolation and treatment, preventing further transmission. The CDC guidelines stress that the first step in an exposure control plan is to recognize patients with signs or risk factors for infectious TB, as unrecognized cases are the primary source of healthcare worker and patient exposures. The Occupational Safety and Health Administration (OSHA) also mandates risk assessment and early detection as foundational to TB control plans.
Option A, "Placement of the patient in an airborne infection isolation room," is a critical control measure once a potentially infectious patient is identified. Airborne infection isolation rooms (AIIRs) with negative pressure ventilation reduce the spread of infectious droplets, as recommended by the CDC. However, this step depends on prior identification; placing a patient in an AIIR without knowing their infectious status is inefficient and not the initial priority. Option C, "Prompt initiation of chemotherapeutic agents," is essential for treating active TB and reducing infectiousness, typically within days of effective therapy, per CDC guidelines. However, this follows identification and diagnosis (e.g., via acid-fast bacilli smear or culture), making it a secondary action rather than the most important preventive aspect. Option D, "Use of personal protective equipment," such as N95 respirators, is a key protective measure for healthcare workers once an infectious patient is identified, as outlined by the CDC and OSHA. However, PPE is a reactive measure that mitigates exposure after identification and isolation, not the foundational step to prevent it.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize early identification as the cornerstone of TB exposure prevention, enabling all subsequent interventions. Option B ensures that the exposure control plan addresses the source of transmission at its outset, making it the most important aspect.
Which of the following is the BEST aid in the identification of patients affected by a recall due to failures in endoscope reprocessing?
Maintaining a log of endoscope use by date of procedure
Maintaining a log of patient identifiers linked with endoscope used
Searching electronic records for endoscope serial number recorded in patient records
Searching electronic records using diagnostic coding to identify all patients that had endoscopy procedures
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes the importance of traceability in endoscope reprocessing programs to ensure rapid and accurate patient notification when reprocessing failures or recalls occur. The most effective method for identifying affected patients is maintaining a log that directly links each endoscope to specific patient identifiers for every procedure.
This type of tracking system allows infection preventionists to quickly determine exactly which patients were exposed to a particular endoscope during the time period of concern. When reprocessing failures are identified—such as incomplete cleaning, high-level disinfection errors, or equipment malfunction—precise linkage between the endoscope and the patient is essential to limit the scope of exposure investigations, reduce unnecessary notifications, and ensure timely follow-up care.
Option A is insufficient because a date-only log does not identify individual patients. Option C may be useful if serial numbers are consistently documented in the medical record, but this practice is not reliably implemented in many facilities and is therefore less dependable. Option D is overly broad and would identify all patients who underwent endoscopy, rather than those exposed to a specific device, leading to unnecessary alarm and inefficient investigations.
For CIC® exam purposes, understanding that patient-to-device linkage logs are the cornerstone of effective exposure investigation and recall management in endoscope reprocessing is critical and aligns with best-practice infection prevention standards.
An infection preventionist (IP) is reviewing blood cultures and notices several results with Arcanobacterium, coagulase-negative Staphylococcus, and Corynebacterium. What action is needed from the IP?
Disregard the results.
Call the Medical Staff Officer and declare there is an outbreak.
Work up each case as a healthcare-acquired bloodstream infection.
Collaborate with the lab manager to determine if there are trends or changes in practice.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that certain organisms commonly recovered from blood cultures—such as Arcanobacterium, coagulase-negative Staphylococcus, and Corynebacterium—are frequently associated with skin contamination rather than true bloodstream infection. When multiple blood cultures yield these organisms, the infection preventionist must assess whether the findings represent contamination related to collection practices rather than immediately assuming infection or outbreak.
The most appropriate action is to collaborate with the laboratory manager and clinical teams to evaluate potential trends, specimen collection techniques, and changes in practice. This includes reviewing blood culture contamination rates, assessing skin antisepsis procedures, evaluating staff competency, and determining whether there has been an increase associated with a specific unit, shift, or collection method. Surveillance data and laboratory quality indicators are essential tools in this evaluation.
Option A is incorrect because results should never be disregarded without assessment. Option B is premature, as the organisms listed are not typical outbreak pathogens and require further analysis before escalation. Option C is inappropriate because these organisms do not automatically meet criteria for healthcare-associated bloodstream infection without supporting clinical evidence.
This scenario reflects a core CIC® exam concept: infection preventionists must apply epidemiologic principles, collaborate with laboratory services, and use data-driven analysis to differentiate contamination from infection and to guide quality improvement efforts.
=========
The BEST choice for surgical instrument cleaning and material compatibility is a detergent solution with:
An acidic pH
A neutral pH
Sodium hypochlorite
Quaternary ammonium compounds
The Certification Study Guide (6th edition) emphasizes that the primary goal of surgical instrument cleaning is to remove organic and inorganic soil while preserving the integrity and functionality of the instrument. For this reason, detergents with a neutral pH are considered the best choice for routine surgical instrument cleaning and material compatibility.
Neutral pH detergents are effective at removing blood, tissue, and other organic matter without causing corrosion, pitting, or degradation of metals, plastics, seals, and coatings commonly used in surgical instruments. The study guide notes that repeated exposure to harsh chemical environments can damage instruments, compromise device performance, and shorten instrument lifespan—ultimately affecting patient safety and increasing replacement costs.
Acidic detergents may be used selectively for removal of mineral deposits or water scale but are not appropriate for routine cleaning due to their corrosive potential. Sodium hypochlorite (bleach) is strongly discouraged for surgical instruments because it is highly corrosive and can rapidly damage stainless steel. Quaternary ammonium compounds are low-level disinfectants and are not suitable for cleaning critical or semi-critical medical devices prior to disinfection or sterilization.
This question reflects a high-yield CIC exam principle: effective cleaning must balance soil removal with material compatibility. Neutral pH detergents best meet both requirements and are widely recommended by manufacturers and reprocessing standards for surgical instrumentation.
Which of the following represents the most effective strategy for preventing Clostridioides difficile transmission in a healthcare facility?
Daily environmental cleaning with quaternary ammonium compounds.
Strict antimicrobial stewardship to limit unnecessary antibiotic use.
Universal C. difficile screening on admission for high-risk patients.
Routine use of alcohol-based hand rub for hand hygiene after patient contact.
Antimicrobial stewardship is the most effective strategy to reduce C. difficile infections (CDI) by limiting the use of broad-spectrum antibiotics.
Quaternary ammonium disinfectants (A) are ineffective against C. difficile spores; bleach-based disinfectants are preferred.
Routine screening (C) is not cost-effective for prevention.
Alcohol-based hand rubs (D) do not kill C. difficile spores; soap and water should be used.
CBIC Infection Control References:
APIC Text, "C. difficile Prevention Strategies," Chapter 9.
What should an infection preventionist prioritize when designing education programs?
Marketing research
Departmental budgets
Prior healthcare experiences
Learning and behavioral science theories
The correct answer is D, "Learning and behavioral science theories," as this is what an infection preventionist (IP) should prioritize when designing education programs. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, effective education programs in infection prevention and control are grounded in evidence-based learning theories and behavioral science principles. These theories, such as adult learning theory (andragogy), social learning theory, and the health belief model, provide a framework for understanding how individuals acquire knowledge, develop skills, and adopt behaviors (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.1 - Develop and implement educational programs). Prioritizing these theories ensures that educational content is tailored to the learners’ needs, enhances engagement, and promotes sustained behavior change—such as adherence to hand hygiene or proper use of personal protective equipment (PPE)—which are critical for reducing healthcare-associated infections (HAIs).
Option A (marketing research) is more relevant to commercial strategies and audience targeting outside the healthcare education context, making it less applicable to the IP’s role in designing clinical education programs. Option B (departmental budgets) is an important logistical consideration for resource allocation, but it is secondary to the design process; financial constraints should influence implementation rather than the foundational design based on learning principles. Option C (prior healthcare experiences) can inform the customization of content by identifying learners’ backgrounds, but it is not the primary priority; it should be assessed within the context of applying learning and behavioral theories to address those experiences effectively.
The focus on learning and behavioral science theories aligns with CBIC’s emphasis on developing and evaluating educational programs that drive measurable improvements in infection control practices (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). By prioritizing these theories, the IP can create programs that are scientifically sound, learner-centered, and impactful, ultimately enhancing patient and staff safety.
An infection preventionist is notified of a patient with Gram negative diplococci from a cerebral spinal fluid specimen. The patient was intubated during ambulance transport and intravenous lines are placed after arrival to the Emergency Department (ED). The patient was immediately placed in Droplet Precautions upon admission to the ED. Which of the following statements is true regarding the need for evaluating exposure to communicable illness?
Follow-up evaluation is not required for this laboratory finding.
ED personnel should be evaluated for possible exposure.
Ambulance personnel should be evaluated for possible exposure.
Follow-up evaluation is not necessary as the appropriate precautions were promptly instituted.
The correct answer is C, "Ambulance personnel should be evaluated for possible exposure," as this statement is true regarding the need for evaluating exposure to communicable illness. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the presence of Gram negative diplococci in a cerebral spinal fluid (CSF) specimen is suggestive of a serious bacterial infection, most likely Neisseria meningitidis, which causes meningococcal disease. This condition is highly contagious and can be transmitted through respiratory droplets or direct contact with respiratory secretions, particularly during procedures like intubation (CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.1 - Identify infectious disease processes). The patient was intubated during ambulance transport, creating a potential aerosol-generating procedure (AGP) that could have exposed ambulance personnel to infectious droplets before Droplet Precautions were instituted upon arrival at the Emergency Department (ED). Therefore, evaluating ambulance personnel for possible exposure is necessary to assess their risk and determine if post-exposure prophylaxis (e.g., antibiotics) or monitoring is required.
Option A (follow-up evaluation is not required for this laboratory finding) is incorrect because the identification of Gram negative diplococci in CSF is a critical finding that warrants investigation due to the potential for meningococcal disease, a reportable and transmissible condition. Option B (ED personnel should be evaluated for possible exposure) is less applicable since the patient was immediately placed in Droplet Precautions upon ED admission, minimizing exposure risk to ED staff after that point, though it could be considered if exposure occurred before precautions were fully implemented. Option D (follow-up evaluation is not necessary as the appropriate precautions were promptly instituted) is inaccurate because the prompt institution of Droplet Precautions in the ED does not retroactively address the exposure risk during ambulance transport, where precautions were not in place.
The focus on evaluating ambulance personnel aligns with CBIC’s emphasis on identifying and mitigating transmission risks associated with communicable diseases, particularly in high-risk settings like ambulance transport (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents). This step is supported by CDC guidelines, which recommend exposure evaluation and prophylaxis for close contacts of meningococcal disease cases (CDC Meningococcal Disease Management, 2021).
An infection preventionist (IP) is informed of a measles outbreak in a nearby community. What is the IP’s FIRST priority when working with Occupational Health?
Isolate employees who have recently traveled to areas with measles outbreaks.
Reassign employees who are pregnant from caring for patients with suspected measles.
Verify that employees in high-risk exposure areas of the facility have adequate immunity to measles.
Set up a mandatory vaccination clinic in collaboration with Occupational Health and local public health partners.
When an infection preventionist (IP) is informed of a measles outbreak in a nearby community, the immediate priority is to protect healthcare workers and patients from potential exposure, particularly in a healthcare setting where vulnerable populations are present. Working with Occupational Health, the IP must follow a structured approach to mitigate the risk of transmission, guided by principles from the Certification Board of Infection Control and Epidemiology (CBIC) and public health guidelines. Let’s evaluate each option to determine the first priority:
A. Isolate employees who have recently traveled to areas with measles outbreaks: Isolating employees who may have been exposed to measles during travel is an important infection control measure to prevent transmission within the facility. However, this action assumes that exposure has already occurred and requires identification of affected employees first. Without knowing the immunity status of the workforce, this step is reactive rather than preventive and cannot be the first priority.
B. Reassign employees who are pregnant from caring for patients with suspected measles: Reassigning pregnant employees is a protective measure due to the severe risks measles poses to fetuses (e.g., congenital rubella syndrome risks, though measles itself is more about maternal complications). This action is specific to a subset of employees and depends on identifying patients with suspected measles, which may not yet be confirmed. It is a secondary step that follows assessing overall immunity and exposure risks, making it inappropriate as the first priority.
C. Verify that employees in high-risk exposure areas of the facility have adequate immunity to measles: Verifying immunity is the foundational step in preventing measles transmission in a healthcare setting. Measles is highly contagious, and healthcare workers in high-risk areas (e.g., emergency departments, pediatric wards) are at increased risk of exposure. The CBIC and CDC recommend ensuring that all healthcare personnel have documented evidence of measles immunity (e.g., two doses of MMR vaccine, laboratory evidence of immunity, or prior infection) as a primary infection control strategy during outbreaks. This step allows the IP to identify vulnerable employees, implement targeted interventions, and comply with occupational health regulations. It is the most proactive and immediate priority when an outbreak is reported in the community.
D. Set up a mandatory vaccination clinic in collaboration with Occupational Health and local public health partners: Establishing a vaccination clinic is a critical long-term strategy to increase immunity and control the outbreak. However, this requires planning, resource allocation, and coordination, which take time. It is a subsequent step that follows verifying immunity status to identify those who need vaccination. While important, it cannot be the first priority due to its logistical demands.
The first priority is C, as verifying immunity among employees in high-risk areas establishes a baseline to prevent transmission before reactive measures (e.g., isolation, reassignment) or broader interventions (e.g., vaccination clinics) are implemented. This aligns with CBIC’s focus on proactive risk assessment and occupational health safety during infectious disease outbreaks, ensuring a rapid response to protect the healthcare workforce and patients.
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain III: Prevention and Control of Infectious Diseases, which prioritizes immunity verification during outbreaks.
CBIC Examination Content Outline, Domain IV: Environment of Care, which includes ensuring employee immunity as part of outbreak preparedness.
CDC Guidelines for Measles Prevention (2023), which recommend verifying healthcare worker immunity as the initial step during a measles outbreak.
A patient with a non-crusted rash has boon diagnosed with Sarcoptes scabiei. The patient is treated with 5% permethrin and precautions are started. The precautions can be stopped
when the treatment cream is applied
when the bed linen is changed
24 hours after effective treatment
24 hours after the second treatment
For Sarcoptes scabiei (scabies), Contact Precautions should remain in place until 24 hours after effective treatment has been completed. The first-line treatment is 5% permethrin cream, which is applied to the entire body and left on for 8–14 hours before being washed off.
Why the Other Options Are Incorrect?
A. When the treatment cream is applied – The mite is still present and infectious until treatment has fully taken effect.
B. When the bed linen is changed – While changing linens is necessary, it does not indicate that the infestation has cleared.
D. 24 hours after the second treatment – Most cases require only one treatment with permethrin, though severe cases may need a second dose after a week.
CBIC Infection Control Reference
According to APIC guidelines, Contact Precautions can be discontinued 24 hours after effective treatment has been administered.
When implementing a multimodal strategy (or bundle) for improving hand hygiene, the infection preventionist should focus on Calculator
signage for hand hygiene reminders.
cost effectiveness of hand hygiene products.
availability of gloves in the patient care area
institutional assessment of significant barriers.
When implementing a multimodal strategy (or bundle) for hand hygiene, the infection preventionist should first assess barriers to compliance before implementing solutions.
Step-by-Step Justification:
Understanding Barriers First:
Identifying barriers (e.g., lack of access to sinks, high workload, or poor compliance culture) is critical for effective intervention.
APIC Guidelines on Hand Hygiene Improvement:
Strategies must be tailored based on the institution's specific challenges.
Why Other Options Are Incorrect:
A. Signage for hand hygiene reminders:
Signage alone is insufficient without addressing systemic barriers.
B. Cost-effectiveness of hand hygiene products:
While important, cost analysis comes after identifying compliance barriers.
C. Availability of gloves in the patient care area:
Gloves do not replace hand hygiene and may lead to lower compliance.
CBIC Infection Control References:
APIC/JCR Workbook, "Hand Hygiene Compliance and Institutional Barriers".
APIC Text, "Hand Hygiene Improvement Strategies".
A 17-year-old presents to the Emergency Department with fever, stiff neck, and vomiting. A lumbar puncture is done. The Gram stain shows Gram negative diplocooci. Presumptive identification of the organism is
Haemophilus influenzae
Neisseria meningitidis
Listeria monocytogenes
Streptococcus pneumoniae
The Gram stain showing Gram-negative diplococci in cerebrospinal fluid (CSF) is characteristic of Neisseria meningitidis, a leading cause of bacterial meningitis in adolescents and young adults.
Step-by-Step Justification:
Gram Stain Interpretation:
Gram-negative diplococci in CSF strongly suggest Neisseria meningitidis.
Classic Symptoms of Meningitis:
Fever, stiff neck, and vomiting are hallmark signs of meningococcal meningitis.
Neisseria meningitidis vs. Other Bacteria:
Haemophilus influenzae (Option A) → Gram-negative coccobacilli.
Listeria monocytogenes (Option C) → Gram-positive rods.
Streptococcus pneumoniae (Option D) → Gram-positive diplococci.
CBIC Infection Control References:
APIC Ready Reference for Microbes, "Neisseria meningitidis and Meningitis".
When conducting a literature search which of the following study designs may provide the best evidence of a direct causal relationship between the experimental factor and the outcome?
A case report
A descriptive study
A case control study
A randomized-controlled trial
To determine the best study design for providing evidence of a direct causal relationship between an experimental factor and an outcome, it is essential to understand the strengths and limitations of each study design listed. The goal is to identify a design that minimizes bias, controls for confounding variables, and establishes a clear cause-and-effect relationship.
A. A case report: A case report is a detailed description of a single patient or a small group of patients with a particular condition or outcome, often including the experimental factor of interest. While case reports can generate hypotheses and highlight rare occurrences, they lack a control group and are highly susceptible to bias. They do not provide evidence of causality because they are observational and anecdotal in nature. This makes them the weakest design for establishing a direct causal relationship.
B. A descriptive study: Descriptive studies, such as cross-sectional or cohort studies, describe the characteristics or outcomes of a population without manipulating variables. These studies can identify associations between an experimental factor and an outcome, but they do not establish causality due to the absence of randomization or control over confounding variables. For example, a descriptive study might show that a certain infection rate is higher in a group exposed to a specific factor, but it cannot prove the factor caused the infection without further evidence.
C. A case control study: A case control study compares individuals with a specific outcome (cases) to those without (controls) to identify factors that may contribute to the outcome. This retrospective design is useful for studying rare diseases or outcomes and can suggest associations. However, it is prone to recall bias and confounding, and it cannot definitively prove causation because the exposure is not controlled or randomized. It is stronger than case reports or descriptive studies but still falls short of establishing direct causality.
D. A randomized-controlled trial (RCT): An RCT is considered the gold standard for establishing causality in medical and scientific research. In an RCT, participants are randomly assigned to either an experimental group (exposed to the factor) or a control group (not exposed or given a placebo). Randomization minimizes selection bias and confounding variables, while the controlled environment allows researchers to isolate the effect of the experimental factor on the outcome. The ability to compare outcomes between groups under controlled conditions provides the strongest evidence of a direct causal relationship. This aligns with the principles of evidence-based practice, which the CBIC (Certification Board of Infection Control and Epidemiology) emphasizes for infection prevention and control strategies.
Based on this analysis, the randomized-controlled trial (D) is the study design that provides the best evidence of a direct causal relationship. This conclusion is consistent with the CBIC's focus on high-quality evidence to inform infection control practices, as RCTs are prioritized in the hierarchy of evidence for establishing cause-and-effect relationships.
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated guidelines, 2023), which emphasizes the use of high-quality evidence, including RCTs, for validating infection control interventions.
CBIC Examination Content Outline, Domain I: Identification of Infectious Disease Processes, which underscores the importance of evidence-based study designs in infection control research.
Which of the following reasons BEST describes the importance of documenting cleaning, disinfection, and sterilization processes?
Reduce the cost of hospital operations.
Ensure compliance with Spaulding classification scheme.
Ensure that all processes are conducted on a regular basis.
Comply with policies, regulations, and accreditation standards.
The Certification Study Guide (6th edition) emphasizes that documentation of cleaning, disinfection, and sterilization processes is a fundamental requirement for regulatory compliance and patient safety assurance. Accurate and complete documentation demonstrates that reprocessing activities are performed according to established policies, manufacturer instructions for use (IFUs), and evidence-based standards. This documentation is essential for meeting expectations set by regulatory agencies, accrediting bodies, and internal quality assurance programs.
Documentation provides verifiable proof that critical steps—such as cleaning, monitoring of sterilization parameters, load release, and equipment maintenance—have been performed correctly. In the event of a healthcare-associated infection investigation, recall, or survey, records serve as objective evidence that proper reprocessing practices were followed. The study guide highlights that “if it is not documented, it is considered not done”, a principle commonly tested on the CIC exam.
The other options reflect secondary or indirect benefits but do not represent the primary reason for documentation. Cost reduction is not the intent of reprocessing records. While Spaulding classification informs how items should be reprocessed, documentation alone does not ensure compliance with that framework. Ensuring processes occur regularly is an operational issue rather than a documentation purpose.
CIC exam questions frequently reinforce that documentation supports accountability, traceability, regulatory compliance, and accreditation readiness, making compliance with policies, regulations, and standards the best answer.
A task force formed to focus on Clostridioides difficile infections (CDIs). The topic of the meeting discussed selecting the correct germicidal wipe. What important factor does the infection preventionist review?
Cost of a case of wipes
Size of individual wipes
Time the surface remains wet
Correct disposal of the wipe
The correct answer is C, "Time the surface remains wet," as this is the most important factor the infection preventionist (IP) should review when selecting a germicidal wipe for controlling Clostridioides difficile infections (CDIs). According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, effective environmental cleaning is a critical component of infection prevention, particularly for pathogens like C. difficile, which forms hardy spores that are resistant to many disinfectants. The efficacy of a germicidal wipe depends on the contact time—the duration the surface must remain wet with the disinfectant to ensure the killing of C. difficile spores. This is specified by the manufacturer and supported by guidelines from the Centers for Disease Control and Prevention (CDC) and the Environmental Protection Agency (EPA), which emphasize that the disinfectant must remain wet on the surface for the full recommended contact time (typically 1-10 minutes for sporicidal agents) to achieve the desired level of disinfection (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols).
Option A (cost of a case of wipes) is a practical consideration for budgeting but is secondary to efficacy in infection control, especially for a high-priority pathogen like C. difficile. Option B (size of individual wipes) may affect coverage and convenience but does not directly impact the wipe’s ability to eliminate the pathogen. Option D (correct disposal of the wipe) is important for preventing cross-contamination and ensuring compliance with waste management protocols, but it is a procedural step after use and not the primary factor in selecting the wipe.
The IP’s review of contact time aligns with CBIC’s focus on evidence-based practices to prevent healthcare-associated infections (HAIs). For C. difficile, which is a leading cause of HAIs, selecting a wipe with an appropriate sporicidal agent and ensuring adequate wet contact time is essential to disrupt transmission, particularly in outbreak settings (CDC Guidelines for Environmental Infection Control in Healthcare Facilities, 2019). This factor directly influences the wipe’s effectiveness, making it the critical review point for the task force.
When a Staphylococcus aureus outbreak is suspected, cultures of which of the following sites will MOST likely identify carriers?
Nose
Hands
Throat
Rectum
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies the anterior nares (nose) as the most common and reliable site for colonization with Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus (MRSA). During suspected outbreaks, culturing the nares is the most effective method for identifying persistent carriers, particularly among healthcare personnel or patients who may serve as reservoirs for transmission.
Nasal carriage of S. aureus is well established in epidemiologic literature and infection prevention practice. Individuals may be persistent carriers, intermittent carriers, or non-carriers, with persistent nasal carriers posing the highest risk for transmission and subsequent infection. The Study Guide emphasizes that nasal colonization strongly correlates with both endogenous infection risk and spread to others, making it the preferred screening site during outbreak investigations.
Hands (Option B) may transiently harbor S. aureus, but hand contamination is temporary and highly variable, making it less useful for identifying long-term carriers. Throat (Option C) and rectum (Option D) are not primary colonization sites for S. aureus and are not routinely used in outbreak screening unless specifically indicated by epidemiologic data.
For CIC® exam purposes, this question reinforces a core infection prevention principle: the anterior nares are the primary reservoir for Staphylococcus aureus, and nasal cultures are the most effective method for identifying carriers during outbreak investigations.
Microfiber cloths and mops are preferred over cotton because microfiber:
Is more cost effective.
Is positively charged to better attract dirt.
Can be laundered and dried with other textiles.
Is versatile for both smooth and rough surfaces.
The CBIC Certified Infection Control Exam Study Guide (6th edition) explains that microfiber cleaning materials are preferred over traditional cotton cloths and mops because of their electrostatic properties, which enhance cleaning effectiveness. Microfiber is composed of very fine synthetic fibers that become positively charged, allowing them to attract and trap negatively charged dirt, dust, and microorganisms rather than simply pushing them across surfaces.
This electrostatic attraction enables microfiber to remove a significantly higher percentage of bacteria and organic material from surfaces compared to cotton, even when used with less cleaning solution or disinfectant. The split fiber structure also increases surface area, allowing microorganisms and debris to be captured within the fibers rather than redistributed. These properties make microfiber particularly effective for environmental cleaning in healthcare settings, where surface contamination contributes to transmission of healthcare-associated infections.
Option A is incorrect because microfiber products are often more expensive initially, though they may be cost-effective over time. Option C is incorrect because microfiber must be laundered separately under specific conditions to maintain effectiveness. Option D may be true but is not the primary reason for preference.
For the CIC® exam, it is important to recognize that microfiber’s positive charge and superior ability to attract and retain microorganisms are the key reasons it is favored over cotton for environmental cleaning and infection prevention.
An infection preventionist (IP) receives a phone call from a local health department alerting the hospital of the occurrence of a sewer main break. Contamination of the city water supply is a possibility. Which of the following actions should the IP perform FIRST?
Notify the Emergency and Admissions departments to report diarrhea cases to infection control.
Review microbiology laboratory reports for enteric organisms in the past week.
Contact the Employee Health department and ask for collaboration in case-finding.
Review the emergency preparedness plan with engineering for sources of potable water.
The correct answer is B, "Review microbiology laboratory reports for enteric organisms in the past week," as this is the first action the infection preventionist (IP) should perform following the alert of a sewer main break and potential contamination of the city water supply. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, a rapid assessment of existing data is a critical initial step in investigating a potential waterborne outbreak. Reviewing microbiology laboratory reports for enteric organisms (e.g., Escherichia coli, Salmonella, or Shigella) helps the IP identify any recent spikes in infections that could indicate water supply contamination, providing an evidence-based starting point for the investigation (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.2 - Analyze surveillance data). This step leverages available hospital data to assess the scope and urgency of the situation before initiating broader actions.
Option A (notify the Emergency and Admissions departments to report diarrhea cases to infection control) is an important subsequent step to enhance surveillance, but it relies on proactive reporting and does not provide immediate evidence of an ongoing issue. Option C (contact the Employee Health department and ask for collaboration in case-finding) is valuable for involving additional resources, but it should follow the initial data review to prioritize case-finding efforts based on identified trends. Option D (review the emergency preparedness plan with engineering for sources of potable water) is a critical preparedness action, but it is more relevant once contamination is confirmed or as a preventive measure, not as the first step in assessing the current situation.
The focus on reviewing laboratory reports aligns with CBIC’s emphasis on using surveillance data to guide infection prevention responses, enabling the IP to quickly determine if the sewer main break has already impacted patient health and to escalate actions accordingly (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms). This approach is consistent with CDC guidelines for responding to waterborne outbreak alerts (CDC Environmental Public Health Guidelines, 2020).
What inflammatory reaction may occur in the eye after cataract surgery due to a breach in disinfection and sterilization of intraocular surgical instruments?
Endophthalmitis
Bacterial conjunctivitis
Toxic Anterior Segment Syndrome
Toxic Posterior Segment Syndrome
The correct answer is C, "Toxic Anterior Segment Syndrome," as this is the inflammatory reaction that may occur in the eye after cataract surgery due to a breach in disinfection and sterilization of intraocular surgical instruments. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, Toxic Anterior Segment Syndrome (TASS) is a sterile, acute inflammatory reaction that can result from contaminants introduced during intraocular surgery, such as endotoxins, residues from improper cleaning, or chemical agents left on surgical instruments due to inadequate disinfection or sterilization processes (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). TASS typically presents within 12-48 hours post-surgery with symptoms like pain, redness, and anterior chamber inflammation, and it is distinct from infectious causes because it is not microbial in origin. A breach in reprocessing protocols, such as failure to remove detergents or improper sterilization, is a known risk factor, making it highly relevant to infection prevention efforts in surgical settings.
Option A (endophthalmitis) is an infectious inflammation of the internal eye structures, often caused by bacterial or fungal contamination, which can also result from poor sterilization but is distinguished from TASS by its infectious nature and longer onset (days to weeks). Option B (bacterial conjunctivitis) affects the conjunctiva and is typically a surface infection unrelated to intraocular surgery or sterilization breaches of surgical instruments. Option D (toxic posterior segment syndrome) is not a recognized clinical entity in the context of cataract surgery; inflammation in the posterior segment is more commonly associated with infectious endophthalmitis or other conditions, not specifically linked to reprocessing failures.
The focus on TASS aligns with CBIC’s emphasis on ensuring safe reprocessing to prevent adverse outcomes in surgical patients, highlighting the need for rigorous infection control measures (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This is supported by CDC and American Academy of Ophthalmology guidelines, which identify TASS as a preventable complication linked to reprocessing errors (CDC Guidelines for Disinfection and Sterilization, 2019; AAO TASS Task Force Report, 2017).
There has been an outbreak of foodborne illness in the community believed to be associated with attendance at a church festival. Which of the following is the MOST appropriate denominator for calculation of the attack rate?
People admitted to hospitals with gastrointestinal symptoms
Admission tickets sold to the festival
Dinners served at the festival
Residents in the county who attended the festival
The attack rate, a key epidemiological measure in outbreak investigations, is defined as the proportion of individuals who become ill after exposure to a suspected source, calculated as the number of cases divided by the population at risk. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes accurate outbreak analysis in the "Surveillance and Epidemiologic Investigation" domain, aligning with the Centers for Disease Control and Prevention (CDC) "Principles of Epidemiology in Public Health Practice" (3rd Edition, 2012). The question involves a foodborne illness outbreak linked to a church festival, requiring the selection of the most appropriate denominator to reflect the population at risk.
Option D, "Residents in the county who attended the festival," is the most appropriate denominator. The attack rate should be based on the total number of people exposed to the potential source of the outbreak (i.e., the festival), as this represents the population at risk for developing the foodborne illness. The CDC guidelines for foodborne outbreak investigations recommend using the number of attendees or participants as the denominator when the exposure is tied to a specific event, such as a festival. This approach accounts for all individuals who had the opportunity to consume the implicated food, providing a comprehensive measure of risk. Obtaining an accurate count of attendees may involve festival records, surveys, or estimates, but it directly reflects the exposed population.
Option A, "People admitted to hospitals with gastrointestinal symptoms," is incorrect as a denominator. This represents the number of cases (the numerator), not the total population at risk. Using cases as the denominator would invalidate the attack rate calculation, which requires a distinct population base. Option B, "Admission tickets sold to the festival," could serve as a proxy for attendees if all ticket holders attended, but it may overestimate the at-risk population if some ticket holders did not participate or underestimate it if additional guests attended without tickets. The CDC advises using actual attendance data when available, making this less precise than Option D. Option C, "Dinners served at the festival," is a potential exposure-specific denominator if the illness is linked to a particular meal. However, without confirmation that all cases are tied to a single dinner event (e.g., a specific food item), this is too narrow and may exclude attendees who ate other foods or did not eat but were exposed (e.g., via cross-contamination), making it less appropriate than the broader attendee count.
The CBIC Practice Analysis (2022) and CDC guidelines stress the importance of defining the exposed population accurately for attack rate calculations in foodborne outbreaks. Option D best captures the population at risk associated with festival attendance, making it the most appropriate denominator.
An infection preventionist is utilizing the Shewhart/Deming cycle in an infection control program performance improvement project. In which of the following steps are the results of the interventions compared with the original goal?
Do
Act
Plan
Study
The correct answer is D, "Study," as this is the step in the Shewhart/Deming cycle (commonly known as the Plan-Do-Study-Act [PDSA] cycle) where the results of the interventions are compared with the original goal. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the PDSA cycle is a systematic approach to quality improvement, widely used in infection control programs to test and refine interventions. The cycle consists of four stages: Plan (designing the intervention and setting goals), Do (implementing the intervention on a small scale), Study (analyzing the data and comparing outcomes against the original goal), and Act (standardizing successful changes or adjusting based on findings) (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). The Study phase is critical for assessing whether the intervention achieved the intended reduction in infection rates or other performance metrics, providing evidence to guide the next steps.
Option A (Do) involves the execution of the planned intervention, focusing on implementation rather than evaluation, so it does not include comparing results. Option B (Act) is the final step where successful interventions are implemented on a broader scale or adjustments are made, but it follows the comparison made in the Study phase. Option C (Plan) is the initial stage of setting objectives and designing the intervention, which occurs before any results are available for comparison.
The emphasis on the Study phase aligns with CBIC’s focus on using data to evaluate the effectiveness of infection prevention strategies, ensuring that performance improvement projects are evidence-based and goal-oriented (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.4 - Evaluate the effectiveness of infection prevention and control interventions). This step enables the infection preventionist to determine if the original goal—such as reducing healthcare-associated infections—was met, facilitating continuous improvement.
An infection preventionist is providing education to a group of medical device reprocessing staff on critical steps in cleaning instruments. Which of the following actions is recommended while using washer-disinfector?
Stack instruments inside the machine
Use circulating water with a pH of 3
Disassemble instruments as much as possible
Close hinged instruments prior to placing in the machine
Best practices for using a washer-disinfector include disassembling instruments and opening hinged instruments to ensure proper cleaning and decontamination.
The APIC Text explains:
“Open hinged instruments and disassemble all instruments… Confirm that spray will be able to reach all loaded items without impedance.”
This ensures water and detergents reach all surfaces. Avoid stacking instruments and ensure proper placement to allow full cleaning.
Which of the following processes is a critical step in sterile reprocessing of surgical instrumentation?
Send sterile processing disposable and reusable instrumentation for sorting.
Wrap instruments in disposable pads for protection until transporting to sterile processing.
Hold dirty instruments until the evening shift to minimize handling before returning to sterile processing.
Remove bioburden at the point of care and keep instrumentation damp until it reaches the sterile processing department.
A critical principle emphasized in the Certification Study Guide (6th edition) is that instrument reprocessing begins at the point of use, not in the sterile processing department. Immediate removal of gross soil and organic material—referred to as bioburden—prevents drying of blood, tissue, and other debris on instruments. Dried organic material significantly interferes with subsequent cleaning, disinfection, and sterilization processes, reducing the effectiveness of these steps and increasing the risk of surgical site infections.
The study guide explains that instruments should be kept moist or damp after use, typically by using an approved enzymatic spray, damp towel, or transport container, to prevent soil from adhering firmly to surfaces and lumens. This practice protects both the integrity of the instruments and the safety of personnel handling them. Delaying cleaning or allowing instruments to dry increases microbial load and biofilm formation, which are difficult to remove during later processing stages.
The incorrect options conflict with infection prevention standards: holding dirty instruments increases contamination risk; wrapping instruments in pads does not address bioburden; and sending instruments for sorting without point-of-care decontamination violates best practices. Proper point-of-care treatment is foundational to safe, effective sterile processing and is consistently tested on the CIC exam.
Hand-hygiene audits in a long-term care facility have demonstrated consistently low levels of staff compliance. An infection preventionist is planning an education program to try to improve hand-hygiene rates. Regarding assessment of the effectiveness of the education program, which of the following is true?
A summative evaluation will accurately reflect the extent to which participants will change their hand-hygiene practices.
Repeated observations of staff will be required in order to demonstrate that the program has been effective.
A change between pre- and post-test scores correlates well with the expected change in hand-hygiene compliance.
An evaluation of the program is not required if the program is mandatory.
The correct answer is B, "Repeated observations of staff will be required in order to demonstrate that the program has been effective," as this statement is true regarding the assessment of the effectiveness of the education program. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, evaluating the impact of an education program on hand-hygiene compliance in a long-term care facility requires ongoing monitoring to assess sustained behavior change. Repeated observations provide direct evidence of staff adherence to hand-hygiene protocols over time, allowing the infection preventionist (IP) to measure the program’s effectiveness beyond initial training (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). This method aligns with the World Health Organization (WHO) and CDC recommendations for hand-hygiene improvement, which emphasize continuous auditing to ensure lasting improvements in compliance rates.
Option A (a summative evaluation will accurately reflect the extent to which participants will change their hand-hygiene practices) is incorrect because a summative evaluation, typically conducted at the end of a program, assesses overall outcomes but does not predict future behavior changes or account for long-term compliance, which is critical in this context. Option C (a change between pre- and post-test scores correlates well with the expected change in hand-hygiene compliance) is misleading; while pre- and post-tests can measure knowledge gain, they do not reliably correlate with actual practice changes, as knowledge does not always translate to behavior without observation. Option D (an evaluation of the program is not required if the program is mandatory) is false, as mandatory programs still require evaluation to verify effectiveness, especially when addressing low compliance, per CBIC and quality improvement standards.
The focus on repeated observations aligns with CBIC’s emphasis on data-driven assessment to improve infection prevention practices, ensuring that the education program leads to sustained hand-hygiene improvements and reduces healthcare-associated infections (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.4 - Evaluate the effectiveness of infection prevention and control interventions).
Which of the following statements is true in considering work reassignment for pregnant employees?
Pregnant employees rarely require work reassignments
Pregnant employees who are positive for hepatitis B surface antibody may not care for hepatitis B patients
Pregnant employees should not be assigned to patients with known infections
Pregnant employees who are not immune to varicella should be excluded from pediatrics
Pregnant healthcare workers who are not immune to varicella (chickenpox) are at increased risk for severe complications if infected. These employees should be excluded from areas like pediatrics where exposure risk is elevated.
The APIC Text specifies:
“Healthcare personnel who are not immune to varicella should avoid exposure to patients with active disease. In high-risk areas such as pediatrics, nonimmune pregnant employees should be reassigned”.
The CIC Study Guide also supports work exclusion or reassignment of nonimmune pregnant staff who have had exposure to varicella or are at risk.
Explanation of incorrect options:
A. Pregnant employees rarely require reassignment – False; reassignment is required in specific high-risk scenarios.
B. Hepatitis B surface antibody positivity means the employee is immune and can care for HBV patients.
C. Broad exclusion from all infected patients is unnecessary and impractical.
A healthcare facility has installed a decorative water fountain in their lobby for the enjoyment of patients and visitors. What is an important issue for the infection preventionist to consider?
Children getting Salmonella enteritidis
Cryptosporidium growth in the fountain
Aerosolization of Legionella pneumophila
Growth of Acinetobacter baumannii
The installation of a decorative water fountain in a healthcare facility lobby introduces a potential environmental hazard that an infection preventionist must evaluate, guided by the Certification Board of Infection Control and Epidemiology (CBIC) principles and infection control best practices. Water features can serve as reservoirs for microbial growth and dissemination, particularly in settings with vulnerable populations such as patients. The key is to identify the most significant infection risk associated with such a water source. Let’s analyze each option:
A. Children getting Salmonella enteritidis: Salmonella enteritidis is a foodborne pathogen typically associated with contaminated food or water sources like poultry, eggs, or untreated drinking water. While children playing near a fountain might theoretically ingest water, Salmonella is not a primary concern for decorative fountains unless they are specifically contaminated with fecal matter, which is uncommon in a controlled healthcare environment. This risk is less relevant compared to other waterborne pathogens.
B. Cryptosporidium growth in the fountain: Cryptosporidium is a parasitic protozoan that causes gastrointestinal illness, often transmitted through contaminated drinking water or recreational water (e.g., swimming pools). While decorative fountains could theoretically harbor Cryptosporidium if contaminated, this organism requires specific conditions (e.g., fecal contamination) and is more associated with untreated or poorly maintained water systems. In a healthcare setting with regular maintenance, this is a lower priority risk compared to bacterial pathogens spread via aerosols.
C. Aerosolization of Legionella pneumophila: Legionella pneumophila is a gram-negative bacterium that thrives in warm, stagnant water environments, such as cooling towers, hot water systems, and decorative fountains. It causes Legionnaires’ disease, a severe form of pneumonia, and Pontiac fever, both transmitted through inhalation of contaminated aerosols. In healthcare facilities, where immunocompromised patients are present, aerosolization from a water fountain poses a significant risk, especially if the fountain is not regularly cleaned, disinfected, or monitored. The CBIC and CDC highlight Legionella as a critical concern in water management programs, making this the most important issue for an infection preventionist to consider.
D. Growth of Acinetobacter baumannii: Acinetobacter baumannii is an opportunistic pathogen commonly associated with healthcare-associated infections (e.g., ventilator-associated pneumonia, wound infections), often found on medical equipment or skin. While it can survive in moist environments, its growth in a decorative fountain is less likely compared to Legionella, which is specifically adapted to water systems. The risk of Acinetobacter transmission via a fountain is minimal unless it becomes a direct contamination source, which is not a primary concern for this scenario.
The most important issue is C, aerosolization of Legionella pneumophila, due to its potential to cause severe respiratory infections, its association with water features, and the heightened vulnerability of healthcare facility populations. The infection preventionist should ensure the fountain is included in the facility’s water management plan, with regular testing, maintenance, and disinfection to prevent Legionella growth and aerosol spread, as recommended by CBIC and CDC guidelines.
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain IV: Environment of Care, which addresses waterborne pathogens like Legionella in healthcare settings.
CBIC Examination Content Outline, Domain III: Prevention and Control of Infectious Diseases, which includes managing environmental risks such as water fountains.
CDC Toolkit for Controlling Legionella in Common Sources of Exposure (2021), which identifies decorative fountains as a potential source of Legionella aerosolization.
Peripherally inserted central catheter (PICC)-associated bloodstream infections (BSIs) have been increasing over the past four months. Which of the following interventions is MOST likely to have contributed to the increase?
Use of chlorhexidine skin antisepsis during insertion of the PICC
Daily bathing adult intensive care unit patients with chlorhexidine
Replacement of the intravenous administration sets every 72 hours
Use of a positive pressure device on the PICC
Peripherally inserted central catheter (PICC)-associated bloodstream infections (BSIs) are a significant concern in healthcare settings, and identifying factors contributing to their increase is critical for infection prevention. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the "Surveillance and Epidemiologic Investigation" and "Prevention and Control of Infectious Diseases" domains, which align with the Centers for Disease Control and Prevention (CDC) guidelines for preventing intravascular catheter-related infections. The question asks for the intervention most likely to have contributed to the rise in PICC-associated BSIs over four months, requiring an evaluation of each option based on evidence-based practices.
Option C, "Replacement of the intravenous administration sets every 72 hours," is the most likely contributor to the increase. The CDC’s "Guidelines for the Prevention of Intravascular Catheter-Related Infections" (2017) recommend that intravenous administration sets (e.g., tubing for fluids or medications) be replaced no more frequently than every 72-96 hours unless clinically indicated (e.g., contamination or specific therapy requirements). Frequent replacement, such as every 72 hours as a routine practice, can introduce opportunities for contamination during the change process, especially if aseptic technique is not strictly followed. Studies cited in the CDC guidelines, including those by O’Grady et al. (2011), indicate that unnecessary manipulation of catheter systems increases the risk of introducing pathogens, potentially leading to BSIs. A change to a 72-hour replacement schedule, if not previously standard, could explain the observed increase over the past four months.
Option A, "Use of chlorhexidine skin antisepsis during insertion of the PICC," is a recommended practice to reduce BSIs. Chlorhexidine, particularly in a 2% chlorhexidine gluconate with 70% alcohol solution, is the preferred skin antiseptic for catheter insertion due to its broad-spectrum activity and residual effect, as supported by the CDC (2017). This intervention should decrease, not increase, infection rates, making it an unlikely contributor. Option B, "Daily bathing adult intensive care unit patients with chlorhexidine," is another evidence-based strategy to reduce healthcare-associated infections, including BSIs, by decolonizing the skin of pathogens like Staphylococcus aureus. The CDC and SHEA (Society for Healthcare Epidemiology of America) guidelines (2014) endorse chlorhexidine bathing in intensive care units, suggesting it should lower, not raise, BSI rates. Option D, "Use of a positive pressure device on the PICC," aims to prevent catheter occlusion and reduce the need for frequent flushing, which could theoretically decrease infection risk by minimizing manipulation. However, there is no strong evidence linking positive pressure devices to increased BSIs; if improperly used or maintained, they might contribute marginally, but this is less likely than the impact of frequent tubing changes.
The CBIC Practice Analysis (2022) and CDC guidelines highlight that deviations from optimal catheter maintenance practices, such as overly frequent administration set replacements, can increase infection risk. Given the four-month timeframe and the focus on an intervention’s potential negative impact, Option C stands out as the most plausible contributor due to the increased manipulation and contamination risk associated with routine 72-hour replacements.
An infection preventionist should collaborate with a public health agency in primary prevention efforts by:
Conducting outbreak investigations.
Performing surveillance for tuberculosis through tuberculin skin test.
Promoting vaccination of health care workers and patients.
Offering blood and body fluid post-exposure prophylaxis.
Primary prevention focuses on preventing the initial occurrence of disease or injury before it manifests, distinguishing it from secondary (early detection) and tertiary (mitigation of complications) prevention. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the "Prevention and Control of Infectious Diseases" domain, which includes collaboration with public health agencies to implement preventive strategies, aligning with the Centers for Disease Control and Prevention (CDC) framework for infection prevention. The question requires identifying the activity that best fits primary prevention efforts.
Option C, "Promoting vaccination of health care workers and patients," is the correct answer. Vaccination is a cornerstone of primary prevention, as it prevents the onset of vaccine-preventable diseases (e.g., influenza, hepatitis B, measles) by inducing immunity before exposure. The CDC’s "Immunization of Health-Care Personnel" (2011) and "General Recommendations on Immunization" (2021) highlight the role of vaccination in protecting both healthcare workers and patients, reducing community transmission and healthcare-associated infections. Collaboration with public health agencies, which often oversee vaccination campaigns and supply distribution, enhances this effort, making it a proactive primary prevention strategy.
Option A, "Conducting outbreak investigations," is a secondary prevention activity. Outbreak investigations occur after cases are identified to control spread and mitigate impact, focusing on containment rather than preventing initial disease occurrence. The CDC’s "Principles of Epidemiology in Public Health Practice" (3rd Edition, 2012) classifies this as a response to an existing problem. Option B, "Performing surveillance for tuberculosis through tuberculin skin test," is also secondary prevention. Surveillance, including tuberculin skin testing, aims to detect latent or active tuberculosis early to prevent progression or transmission, not to prevent initial infection. The CDC’s "Guidelines for Preventing the Transmission of Mycobacterium tuberculosis" (2005) supports this as a screening tool. Option D, "Offering blood and body fluid post-exposure prophylaxis," is tertiary prevention. Post-exposure prophylaxis (e.g., for HIV or hepatitis B) is administered after potential exposure to prevent disease development, focusing on mitigating consequences rather than preventing initial exposure, as outlined in the CDC’s "Updated U.S. Public Health Service Guidelines" (2013).
The CBIC Practice Analysis (2022) and CDC guidelines prioritize vaccination as a primary prevention strategy, and collaboration with public health agencies amplifies its reach. Option C best reflects this preventive focus, making it the correct choice.
Ongoing education for the Infection Preventionist (IP) is MOST important because
the healthcare environment is fast-paced with frequent changes.
motivation to change comes from the Management Team.
self-directed learning is not a major force for the adult learner.
it is necessary to maintain a competitive edge.
Ongoing education for Infection Preventionists (IPs) is essential due to the rapidly evolving healthcare landscape and emergence of new infectious diseases, regulations, and technologies.
From the APIC Text:
“Professional development is essential to keeping the infection preventionist up to date with the latest knowledge, skills, and strategies for preventing infections.”
The APIC/JCR Workbook also notes:
“Because information related to emerging infectious diseases... changes rapidly... IPs should actively review information for updates and guidance.”
A change in the disinfection protocol is indicated for which of the following scenarios?
A high-level disinfectant being used for diaphragm fitting rings
Sodium hypochlorite being used for blood pressure cuffs
An enzymatic solution being used for rectal probes
2% glutaraldehyde being used for cryosurgical probes
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes the importance of applying Spaulding’s classification to determine appropriate cleaning, disinfection, and sterilization levels for medical devices based on their intended use. According to this framework, rectal probes are classified as semi-critical devices because they come into contact with mucous membranes. Semi-critical devices require at least high-level disinfection after thorough cleaning.
An enzymatic solution, as listed in option C, is not a disinfectant. Enzymatic detergents are designed solely for cleaning, meaning they help remove organic material such as blood, mucus, and feces, but they do not kill microorganisms. Using an enzymatic solution alone for rectal probes is therefore inadequate and represents an improper disinfection practice, making this the scenario that clearly requires a protocol change.
Option A is acceptable because diaphragm fitting rings are noncritical devices that contact intact skin and may be safely processed using high-level disinfection. Option B is appropriate because blood pressure cuffs are noncritical items and can be disinfected using low- to intermediate-level disinfectants such as sodium hypochlorite. Option D is also appropriate, as cryosurgical probes are semi-critical devices and 2% glutaraldehyde is an accepted high-level disinfectant.
Recognizing the distinction between cleaning versus disinfection and applying the correct level of processing is a core competency for infection preventionists and a frequently tested concept on the CIC® exam.
==========
A new hospital disinfectant with a 3-minute contact time has been purchased by Environmental Services. The disinfectant will be rolled out across the patient care 3-minute contact time has been purchased by Environmental Services. The disinfectant will be rolled out across the patient care areas. They are concerned about the high cost of the disinfectant. What advice can the infection preventionist provide?
Use the new disinfectant for patient washrooms only.
Use detergents on the floors in patient rooms.
Use detergents on smooth horizontal surfaces.
Use new disinfectant for all surfaces in the patient room.
The scenario involves the introduction of a new hospital disinfectant with a 3-minute contact time, intended for use across patient care areas, but with concerns raised by Environmental Services about its high cost. The infection preventionist’s advice must balance infection control efficacy with cost management, adhering to principles outlined by the Certification Board of Infection Control and Epidemiology (CBIC) and evidence-based practices. The goal is to optimize the disinfectant’s use while ensuring a safe environment. Let’s evaluate each option:
A. Use the new disinfectant for patient washrooms only: Limiting the disinfectant to patient washrooms focuses its use on high-touch, high-risk areas where pathogens (e.g., Clostridioides difficile, norovirus) may be prevalent. However, this approach restricts the disinfectant’s application to a specific area, potentially leaving other patient care surfaces (e.g., bed rails, tables) vulnerable to contamination. While cost-saving, it does not address the broad infection control needs across all patient care areas, making it an incomplete strategy.
B. Use detergents on the floors in patient rooms: Detergents are cleaning agents that remove dirt and organic material but lack the antimicrobial properties of disinfectants. Floors in patient rooms can harbor pathogens, but they are generally considered lower-risk surfaces compared to high-touch areas (e.g., bed rails, doorknobs). Using detergents instead of the new disinfectant on floors could reduce costs but compromises infection control, as floors may still contribute to environmental transmission (e.g., via shoes or equipment). This option is not optimal given the availability of an effective disinfectant.
C. Use detergents on smooth horizontal surfaces: Smooth horizontal surfaces (e.g., tables, counters, overbed tables) are common sites for pathogen accumulation and transmission in patient rooms. Using detergents to clean these surfaces removes organic material, which is a critical first step before disinfection. If the 3-minute contact time disinfectant is reserved for high-touch or high-risk surfaces (e.g., bed rails, call buttons) where disinfection is most critical, this approach maximizes the disinfectant’s efficacy while reducing its overall use and cost. This strategy aligns with CBIC guidelines, which emphasize a two-step process (cleaning followed by disinfection) and targeted use of resources, making it a practical and cost-effective recommendation.
D. Use new disinfectant for all surfaces in the patient room: Using the disinfectant on all surfaces ensures comprehensive pathogen reduction but increases consumption and cost, which is a concern for Environmental Services. While the 3-minute contact time suggests efficiency, overusing the disinfectant on low-risk surfaces (e.g., floors, walls) may not provide proportional infection control benefits and could strain the budget. This approach does not address the cost concern and is less strategic than targeting high-risk areas.
The best advice is C, using detergents on smooth horizontal surfaces to handle routine cleaning, while reserving the new disinfectant for high-touch or high-risk areas where its antimicrobial action is most needed. This optimizes infection prevention, aligns with CBIC’s emphasis on evidence-based environmental cleaning, and addresses the cost concern by reducing unnecessary disinfectant use. The infection preventionist should also recommend a risk assessment to identify priority surfaces for disinfectant application.
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain IV: Environment of Care, which advocates for targeted cleaning and disinfection based on risk.
CBIC Examination Content Outline, Domain III: Prevention and Control of Infectious Diseases, which includes cost-effective use of disinfectants.
CDC Guidelines for Environmental Infection Control in Healthcare Facilities (2022), which recommend cleaning with detergents followed by targeted disinfection.
In evaluating the infection control and ventilation measures for operating rooms the Infection Preventionist should know that the air changes per hour (ACH) should be maintained at greater than or equal to 15 ACH. How many of these changes should be fresh air?
Greater than or equal to 3
Greater than or equal to 5
Greater than or equal to 6
Greater than or equal to 7
In operating rooms, a minimum of 15 air changes per hour (ACH) is required, with at least 3 of those ACH being from fresh or outdoor air. This requirement helps reduce microbial contamination and provides a clean surgical environment.
According to the APIC Text:
"In each, air should flow out of the room and the minimum ACH should be 15, with three of these ACH being fresh or outdoor air."
This aligns with design specifications outlined in the 2006 Guidelines for design and construction of health care facilities.
Which of the following anti-infective materials is used on endotracheal tubes, urine catheters, and intravascular catheters?
Silver
Copper
Chromium
Zinc
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies silver as an anti-infective material commonly incorporated into medical devices such as endotracheal tubes, urinary catheters, and intravascular catheters. Silver has broad-spectrum antimicrobial properties against bacteria, fungi, and some viruses. When used as a coating or impregnated material, silver ions disrupt microbial cell membranes, interfere with enzyme systems, and inhibit replication—thereby reducing microbial colonization and biofilm formation on device surfaces.
Device-associated infections often originate from colonization of indwelling devices. Silver-coated or silver-impregnated devices are intended to reduce the risk of healthcare-associated infections by limiting early microbial adherence and growth, particularly during the highest-risk period shortly after device insertion. Examples include silver alloy urinary catheters for CAUTI prevention and silver-coated endotracheal tubes designed to reduce ventilator-associated events.
The other options listed are not used in this context. Copper has antimicrobial properties but is not commonly used in indwelling medical devices. Chromium is used for corrosion resistance in alloys, not for infection prevention. Zinc plays roles in wound care and topical formulations but is not standard for catheter or tube coatings.
For CIC® exam preparation, recognizing silver as the anti-infective material used in multiple indwelling devices is important, as it reflects evidence-based strategies aimed at reducing device-associated infection risk.
==========
A facility's goal is to increase hand-hygiene compliance from the current 52% to 75% within 12 months. A gap analysis identifies several different issues. Which of the following is BEST suited for summarizing these issues?
Gantt chart
Flow chart
Ishikawa diagram
Affinity diagram
An Ishikawa diagram (fishbone diagram) is used to visually represent cause-and-effect relationships in problem analysis. It is best for summarizing and categorizing issues found in a gap analysis related to infection prevention.
The APIC Text confirms:
“A fishbone diagram (also called a tree diagram or Ishikawa) allows a team to identify, explore, and graphically display all of the possible causes related to a problem to discover the root cause”.
It’s particularly useful in quality improvement and infection prevention project analysis.
A hospital experiencing an increase in catheter-associated urinary tract infections (CAUTI) implements a quality improvement initiative. Which of the following interventions is MOST effective in reducing CAUTI rates?
Routine urine cultures for all catheterized patients every 48 hours.
Implementing nurse-driven protocols for early catheter removal.
Replacing indwelling urinary catheters with condom catheters for all male patients.
Using antibiotic-coated catheters in all ICU patients.
Nurse-driven catheter removal protocols have been shown to significantly reduce CAUTI rates by minimizing unnecessary catheter use.
Routine urine cultures (A) lead to overtreatment of asymptomatic bacteriuria.
Condom catheters (C) are helpful in certain cases but are not universally effective.
Antibiotic-coated catheters (D) have mixed evidence regarding their effectiveness.
CBIC Infection Control References:
APIC Text, "CAUTI Prevention Strategies," Chapter 10.
An infection preventionist is observing the cleaning and disinfection process of semi-critical devices. To ensure these items have been reprocessed meeting the minimum requirements, which of the following is required?
Use of detergents with pH lower than 7
Initial cleaning must begin 24 hours after use
Initial cleaning must begin as soon as possible after use
Soaking in a solution of liquid chemical sterilant between 3 and 12 hours
The Certification Study Guide (6th edition) emphasizes that thorough cleaning is the most critical step in the reprocessing of all reusable medical devices, including semi-critical devices (those that contact mucous membranes or nonintact skin). A foundational requirement is that initial cleaning begins as soon as possible after use. Prompt cleaning prevents organic material—such as blood, secretions, and tissue—from drying on device surfaces and within lumens, which can shield microorganisms and significantly reduce the effectiveness of subsequent disinfection.
The study guide explains that delayed cleaning increases the risk of biofilm formation and makes removal of soil more difficult, potentially compromising patient safety. For this reason, point-of-use pre-cleaning and rapid transport to reprocessing are considered minimum expectations. Cleaning must occur before any high-level disinfection or sterilization; without effective cleaning, even correctly selected disinfectants may fail.
The other options are incorrect or misleading. There is no universal requirement for detergents with pH lower than 7; detergent selection should follow manufacturer instructions. Waiting 24 hours before cleaning is contrary to best practice and increases risk. Soaking devices in liquid chemical sterilants for extended periods does not address the prerequisite of cleaning and may not be appropriate for semi-critical devices unless specified by the manufacturer.
This question reflects a key CIC exam principle: timely cleaning is non-negotiable and is the cornerstone of safe device reprocessing.
An infection preventionist (IP) is notified that a patient who underwent an endoscopic brain biopsy the night before has been diagnosed with prion disease. Because the diagnosis was thought to be unlikely but possible at the time of the biopsy, the endoscope was sequestered. The endoscope manufacturer’s instructions for reprocessing indicate that the endoscope can be reprocessed using high-level disinfection or low-temperature sterilization. The IP should recommend that the endoscope be:
Bagged as biohazardous waste and discarded.
Autoclaved at 134°C (273°F) for 18 minutes.
Disinfected with a 1:10 dilution of household bleach or 1N NaOH.
Sterilized using ethylene oxide or hydrogen peroxide gas plasma.
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies prion diseases (such as Creutzfeldt-Jakob disease) as unique and extremely challenging from an infection prevention standpoint due to the extraordinary resistance of prions to conventional disinfection and sterilization methods. Prions are not destroyed by standard high-level disinfection, low-temperature sterilization, ethylene oxide, or hydrogen peroxide gas plasma, even when manufacturer instructions for use suggest these methods for routine pathogens.
Invasive neurologic procedures involving high-risk tissues (brain, spinal cord, posterior eye) pose the greatest transmission risk. When a reusable device such as an endoscope is used on high-risk tissue in a patient with known or suspected prion disease, and the device cannot tolerate validated prion-inactivation protocols, the Study Guide recommends removal from service and disposal.
While harsh chemical treatments such as 1N sodium hydroxide or high-concentration bleach combined with extended steam sterilization may be effective for heat-resistant surgical instruments, flexible endoscopes and similar devices cannot safely undergo these processes without damage. Therefore, reprocessing is not acceptable in this scenario.
Autoclaving alone and low-temperature sterilization methods are ineffective against prions. As a result, the safest and recommended action is to bag the device as biohazardous waste and discard it, preventing any risk of iatrogenic transmission.
For the CIC® exam, this question tests recognition that manufacturer IFUs do not supersede prion-specific infection prevention guidance, and patient safety requires device destruction when prion exposure cannot be reliably mitigated.
The Infection Control Department is notified of possible contamination of one lot of dressings. Which of the following actions should be taken?
Instruct the Purchasing Department to remove the manufacturer’s dressings and similar dressings from the hospital.
Notify discharged patients on whom the dressings were used to be alert for signs of infection.
Identify where the implicated dressings are in the hospital so that they can be returned to the manufacturer.
Arrange to purchase new dressings from a different manufacturer.
The Certification Study Guide (6th edition) emphasizes that when a specific product lot is suspected or confirmed to be contaminated, the first priority is containment and traceability. The infection preventionist must promptly identify where the implicated lot is located within the facility so it can be removed from use, quarantined, and managed according to recall or manufacturer instructions. This step prevents further patient exposure and preserves the ability to conduct an accurate risk assessment.
Locating the affected dressings allows the facility to determine how widely the product has been distributed, whether it is still in use, and which clinical areas may be affected. This information is essential before taking additional actions such as patient notification or broad product removal. The study guide stresses that responses must be proportionate and evidence-based, avoiding unnecessary disruption or alarm.
The other options represent actions that may be considered later, depending on findings. Removing all dressings from the same manufacturer is overly broad when only one lot is implicated. Notifying discharged patients is premature unless patient exposure and risk have been confirmed. Purchasing from a different manufacturer does not address the immediate need to control and investigate the current issue.
CIC exam questions often focus on sequencing of actions during product contamination events. Correctly identifying and isolating the affected product lot is the foundational step that enables safe, effective follow-up and regulatory compliance.
At a facility with 10.000 employees. 5,000 are at risk for bloodbome pathogen exposure. Over the past five years, 100 of the 250 needlestick injuries involved exposure to bloodborne pathogens, and 2% of exposed employees seroconverted. How many employees became infected?
1
2
5
10
To determine the number of employees who seroconverted (became infected) after a needlestick exposure, we use the given data:
Total Needlestick Injuries: 250
Needlestick Injuries Involving Bloodborne Pathogens: 100
Seroconversion Rate: 2%
Calculation:
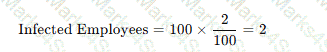
Why Other Options Are Incorrect:
A. 1: Incorrect calculation; 2% of 100 is 2, not 1.
C. 5: Overestimates the actual number of infections.
D. 10: Exceeds the calculated value based on given data.
CBIC Infection Control References:
APIC Text, "Occupational Exposure and Seroconversion Risks".
APIC Text, "Bloodborne Pathogens and Needlestick Injury Prevention"
Which of the following management activities should be performed FIRST?
Evaluate project results
Establish goals
Plan and organize activities
Assign responsibility for projects
To determine which management activity should be performed first, we need to consider the logical sequence of steps in effective project or program management, particularly in the context of infection control as guided by CBIC principles. Management activities typically follow a structured process, and the order of these steps is critical to ensuring successful outcomes.
A. Evaluate project results: Evaluating project results involves assessing the outcomes and effectiveness of a project after its implementation. This step relies on having completed the project or at least reached a stage where outcomes can be measured. Performing this activity first would be premature, as there would be no results to evaluate without prior planning, goal-setting, and execution. Therefore, this cannot be the first step.
B. Establish goals: Establishing goals is the foundational step in any management process. Goals provide direction, define the purpose, and set the criteria for success. In the context of infection control, as emphasized by CBIC, setting clear objectives (e.g., reducing healthcare-associated infections by a specific percentage) is essential before any other activities can be planned or executed. This step aligns with the initial phase of strategic planning, making it the logical first activity. Without established goals, subsequent steps lack focus and purpose.
C. Plan and organize activities: Planning and organizing activities involve developing a roadmap to achieve the goals, including timelines, resources, and tasks. This step depends on having clear goals to guide the planning process. In infection control, this might include designing interventions to meet infection reduction targets. While critical, it cannot be the first step because planning requires a predefined objective to be effective.
D. Assign responsibility for projects: Assigning responsibility involves delegating tasks and roles to individuals or teams. This step follows the establishment of goals and planning, as responsibilities need to be aligned with the specific objectives and organized activities. In an infection control program, this might mean assigning staff to monitor compliance with hand hygiene protocols. Doing this first would be inefficient without a clear understanding of the goals and plan.
The correct sequence in management, especially in a structured field like infection control, begins with establishing goals to provide a clear target. This is followed by planning and organizing activities, assigning responsibilities, and finally evaluating results. The CBIC framework supports this approach by emphasizing the importance of setting measurable goals as part of the infection prevention and control planning process, which is a prerequisite for all subsequent actions.
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain V: Management and Communication, which highlights the importance of setting goals as the initial step in managing infection control programs.
CBIC Examination Content Outline, Domain V: Leadership and Program Management, which underscores the need for goal-setting prior to planning and implementation of infection control initiatives.
Based on the scenarios, when should an infection preventionist suspect an outbreak?
Three positive routine environmental cultures of Staphylococcus aureus from the bone marrow transplant unit
Detection of three ventilator-associated pneumonia cases among patients in the intensive care unit (ICU) after updated case definition implementation
Increase in the number of Klebsiella pneumoniae carbapenemase–producing isolates in the ICU after implementation of new minimum inhibitory concentration breakpoints
Detection of three positive blood cultures with methicillin-resistant Staphylococcus aureus in the cardiac ICU for patients who underwent cardiac surgery in the same week
The Certification Study Guide (6th edition) emphasizes that an outbreak should be suspected when there is an unexpected clustering of infections by time, place, and person, particularly when cases share a common exposure or procedure. Option D meets all key criteria for outbreak suspicion: the same organism (methicillin-resistant Staphylococcus aureus), the same location (cardiac ICU), a common procedure (cardiac surgery), and a tight time frame (same week). This constellation strongly suggests possible transmission related to surgical practices, postoperative care, or shared equipment.
The other scenarios reflect situations that do not necessarily indicate an outbreak. Routine environmental cultures are not recommended for outbreak detection and often do not correlate with patient infection risk. An apparent increase in ventilator-associated pneumonia following implementation of a new case definition is likely due to surveillance artifact, not true transmission. Similarly, increases in carbapenemase-producing Klebsiella pneumoniae after adoption of new laboratory breakpoints reflect diagnostic changes, not an epidemiologic event.
The study guide stresses the importance of distinguishing true outbreaks from pseudo-outbreaks caused by changes in definitions, testing methods, or surveillance intensity. CIC exam questions frequently test this concept. Recognizing a true outbreak requires linking cases through epidemiologic characteristics—not simply increases in numbers.
Prompt recognition of true outbreaks enables timely investigation, implementation of control measures, and prevention of further transmission.
A ventilator-associated pneumonia rate in the ICU has increased from 8.1 infections/1,000 ventilator days to 15.4 infections/1,000 ventilator days over the past two months. To determine the root cause for this increase, the MOST appropriate tool for a performance improvement team is a:
Fishbone diagram
Pareto chart
Flow diagram
Control chart
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies the fishbone diagram, also known as a cause-and-effect diagram or Ishikawa diagram, as the most appropriate tool for conducting root cause analysis when investigating an increase in adverse outcomes such as ventilator-associated pneumonia (VAP). This tool is specifically designed to systematically explore multiple contributing factors that may be driving a problem.
A fishbone diagram helps a multidisciplinary performance improvement team organize potential causes into logical categories, commonly including people, processes, equipment, environment, materials, and policies. In the case of rising VAP rates, the team might examine factors such as ventilator care practices, oral hygiene compliance, head-of-bed elevation, sedation practices, staffing levels, equipment maintenance, and adherence to prevention bundles. By visually mapping these contributors, the team can identify underlying system issues rather than focusing on isolated events or individual performance.
The other tools listed are less appropriate for root cause determination. A Pareto chart is useful for prioritizing the most frequent contributors after causes are identified, but it does not identify causes itself. A flow diagram maps process steps but does not analyze why failures occur. A control chart monitors variation over time but does not explain causation.
For CIC® exam preparation, it is essential to recognize that fishbone diagrams are the primary tool for identifying root causes in performance improvement investigations involving increased infection rates.
There are four cases of ventilator-associated pneumonia in a surgical intensive care unit with a total of 200 ventilator days and a census of 12 patients. Which of the following BEST expresses how this should be reported?
Ventilator-associated pneumonia rate of 2%
20 ventilator-associated pneumonia cases/1000 ventilator days
Postoperative pneumonia rate of 6% in SICU patients
More information is needed regarding ventilator days per patient
The standard way to report ventilator-associated pneumonia (VAP) rates is:
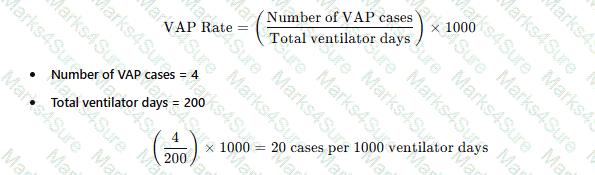
Why the Other Options Are Incorrect?
A. Ventilator-associated pneumonia rate of 2% – This does not use the correct denominator (ventilator days).
C. Postoperative pneumonia rate of 6% in SICU patients – Not relevant, as the data focuses on VAP, not postoperative pneumonia.
D. More information is needed regarding ventilator days per patient – The total ventilator days are already provided, so no additional data is required.
CBIC Infection Control Reference
APIC and NHSN recommend reporting VAP rates as cases per 1,000 ventilator days.
What is the most effective early detection strategy for emerging public health threats?
Visit local, state, and federal public health websites on a regular schedule.
Subscribe to public health alerts at the local, state, and/or federal level.
Attend educational and professional webinars on global outbreaks.
Rely on information provided by the facility laboratory.
Early detection of emerging public health threats depends on receiving timely, actionable information that can trigger rapid assessment and response within the facility. The Certification Study Guide emphasizes preparedness for biologic threats and emerging infectious diseases as part of core infection prevention practice (e.g., planning for an influx of patients with communicable diseases and responding to emerging infections). Subscribing to public health alerts is the most effective option because alerts are designed to push critical updates (case definitions, exposure risks, recommended control measures, and reporting expectations) as soon as they are identified by public health authorities—minimizing delay compared with periodically checking websites.
Why the other options are incorrect:
A is reactive and can miss urgent updates between scheduled checks.
C supports ongoing education but is not a real-time early warning system.
D is important for facility-level detection, but emerging threats are often identified first through public health surveillance and communications beyond a single facility’s lab.
An example of active learning is:
Listening to a lecture.
Reading policies.
Exploring case studies.
Watching a recorded presentation.
Active learning is a core educational principle emphasized in the Education and Research domain of the CBIC Certified Infection Control Exam Study Guide (6th edition). Active learning requires the learner to engage cognitively with the material through analysis, problem-solving, and application of knowledge, rather than passively receiving information. Exploring case studies is a classic example of active learning because it requires participants to apply infection prevention principles to real-world or simulated scenarios, interpret data, evaluate risks, and make evidence-based decisions.
The Study Guide highlights that adult learners—such as infection preventionists and healthcare professionals—retain knowledge more effectively when learning activities are interactive and practice-oriented. Case studies encourage critical thinking by presenting complex clinical or operational situations that mirror challenges encountered in infection prevention practice, such as outbreak investigations, surveillance interpretation, or policy implementation. This method supports deeper understanding and long-term retention.
In contrast, listening to lectures, reading policies, or watching recorded presentations are considered passive learning activities. While these methods are valuable for introducing foundational knowledge or disseminating information, they do not actively involve the learner in applying or synthesizing information. The Study Guide specifically notes that combining passive methods with active strategies—such as case discussions, simulations, and problem-based learning—enhances competency development and performance improvement in infection prevention programs.
This distinction is frequently tested on the CIC® exam, making recognition of active learning strategies essential for exam success.
A 2-yoar-old girl is admitted with a fractured tibia. At birth, she was diagnosed with congenital cytomegalovirus (CMV). Which of the following barrier precautions is appropriate for healthcare personnel caring for her?
Wear masks and gloves
Wear gloves when handling body fluids
No barrier precautions are needed
Use gowns, masks, gloves, and a private room
Standard Precautions are sufficient for congenital cytomegalovirus (CMV), which means that gloves should be used when handling body fluids. CMV is primarily transmitted via direct contact with saliva, urine, or blood.
Why the Other Options Are Incorrect?
A. Wear masks and gloves – Masks are not necessary unless performing high-risk aerosol-generating procedures.
C. No barrier precautions are needed – Gloves are required when handling bodily fluids to prevent transmission.
D. Use gowns, masks, gloves, and a private room – CMV does not require Contact or Airborne Precautions.
CBIC Infection Control Reference
APIC guidelines state that CMV transmission is prevented using Standard Precautions, primarily with glove use for body fluid contact.
An 84-year-old male with a gangrenous foot is admitted to the hospital from an extended-care facility (ECF). The ECF is notified that the wound grew Enterococcus faecium with the following antibiotic sensitivity results:
ampicillin – R
vancomycin – R
penicillin – R
linezolid – S
This is the fourth Enterococcus species cultured from residents within the same ECF wing in the past month. The other cultures were from two urine specimens and a draining wound. The Infection Preventionist (IP) should immediately:
Notify the medical director of the outbreak.
Compare the four culture reports and sensitivity patterns.
Conduct surveillance cultures for this organism in all residents.
Notify the nursing administrator to close the wing to new admissions.
The scenario describes a potential outbreak of multidrug-resistant Enterococcus faecium in an extended-care facility (ECF) wing, indicated by four positive cultures (including the current case and three prior cases from urine and a draining wound) within a month. The organism exhibits resistance to ampicillin, vancomycin, and penicillin, but sensitivity to linezolid, suggesting a possible vancomycin-resistant Enterococcus (VRE) strain, which is a significant concern in healthcare settings. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the importance of rapid outbreak detection and response in the "Surveillance and Epidemiologic Investigation" domain, aligning with Centers for Disease Control and Prevention (CDC) guidelines for managing multidrug-resistant organisms (MDROs).
Option A, "Notify the medical director of the outbreak," is the most immediate and critical action. Identifying an outbreak—defined by the CDC as two or more cases of a similar illness linked by time and place—requires prompt notification to the facility’s leadership (e.g., medical director) to initiate a coordinated response. The presence of four Enterococcus cases, including a multidrug-resistant strain, within a single ECF wing over a month suggests a potential cluster, necessitating urgent action to assess the scope, implement control measures, and allocate resources. The CDC’s "Management of Multidrug-Resistant Organisms in Healthcare Settings" (2006) recommends immediate reporting to facility leadership as the first step to activate an outbreak investigation team, making this the priority.
Option B, "Compare the four culture reports and sensitivity patterns," is an important subsequent step in outbreak investigation. Analyzing the antibiotic susceptibility profiles and culture sources can confirm whether the cases are epidemiologically linked (e.g., clonal spread of VRE) and guide treatment and control strategies. However, this is a detailed analysis that follows initial notification and should not delay alerting the medical director. Option C, "Conduct surveillance cultures for this organism in all residents," is a proactive measure to determine the prevalence of Enterococcus faecium, especially VRE, within the wing. The CDC recommends targeted surveillance during outbreaks, but this requires prior authorization and planning by the outbreak team, making it a secondary action after notification. Option D, "Notify the nursing administrator to close the wing to new admissions," may be a control measure to prevent further spread, as suggested by the CDC for MDRO outbreaks. However, closing a unit is a significant decision that should be guided by the medical director and infection control team after assessing the situation, not an immediate independent action by the IP.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize rapid communication with leadership to initiate a structured outbreak response, including resource allocation and policy adjustments. Given the multidrug-resistant nature and cluster pattern, notifying the medical director (Option A) is the most immediate and appropriate action to ensure a comprehensive response.
An infection preventionist (IP) is tasked with developing an infection prevention training program for family members. What step should the IP take FIRST?
Assess the needs of the family members at the facility.
Create clearly defined goals and objectives for the training.
Ensure that all content in the training is relevant and practical.
Develop a plan to create an appropriate training environment.
The correct answer is A, "Assess the needs of the family members at the facility," as this is the first step the infection preventionist (IP) should take when developing an infection prevention training program for family members. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, effective education programs begin with a needs assessment to identify the specific knowledge gaps, cultural factors, and practical challenges of the target audience—in this case, family members. This initial step ensures that the training is tailored to their level of understanding, language preferences, and the infection risks they may encounter (e.g., hand hygiene, isolation protocols), aligning with adult learning principles (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.1 - Develop and implement educational programs). Without this assessment, subsequent steps risk being misaligned with the audience’s needs, reducing the program’s effectiveness.
Option B (create clearly defined goals and objectives for the training) is a critical step but follows the needs assessment, as goals should be based on identified needs to ensure relevance. Option C (ensure that all content in the training is relevant and practical) depends on understanding the audience’s needs first, making it a later step in the development process. Option D (develop a plan to create an appropriate training environment) is important for implementation but requires prior knowledge of the audience and content to design effectively.
The focus on assessing needs aligns with CBIC’s emphasis on evidence-based education design, enabling the IP to address specific infection prevention priorities for family members and improve outcomes in the facility (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). This approach is supported by CDC guidelines, which recommend audience assessment as a foundational step in health education programs.
The degree of infectiousness of a patient with tuberculosis correlates with
the hand-hygiene habits of the patient.
a presence of acid-fast bacilli in the blood.
a tuberculin skin test result that is greater than 20 mm
the number of organisms expelled into the air
The infectiousness of tuberculosis (TB) is directly related to the number of Mycobacterium tuberculosis organisms expelled into the air by an infected patient.
Step-by-Step Justification:
TB Transmission Mechanism:
TB spreads through airborne droplet nuclei, which remain suspended for long periods.
Factors Affecting Infectiousness:
High bacterial load in sputum: Smear-positive patients are much more infectious.
Coughing and sneezing frequency: More expelled droplets increase exposure risk.
Environmental factors: Poor ventilation increases transmission.
Why Other Options Are Incorrect:
A. Hand hygiene habits: TB is airborne, not transmitted via hands.
B. Presence of acid-fast bacilli (AFB) in blood: TB is not typically hematogenous, and blood AFB does not correlate with infectiousness.
C. Tuberculin skin test (TST) >20 mm: TST indicates prior exposure, not infectiousness.
CBIC Infection Control References:
APIC Text, "Tuberculosis Transmission and Control Measures".
Which of the following is included in an effective respiratory hygiene program in healthcare facilities?
Community educational brochures campaign
Mask availability at building entrance and reception
Separate entrance for symptomatic patients and visitors
Temperature monitoring devices at clinical unit entrance
An effective respiratory hygiene program in healthcare facilities aims to reduce the transmission of respiratory pathogens, such as influenza, COVID-19, and other droplet- or airborne infectious agents, by promoting practices that minimize the spread from infected individuals. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the importance of such programs within the "Prevention and Control of Infectious Diseases" domain, aligning with guidelines from the Centers for Disease Control and Prevention (CDC). The CDC’s "Guideline for Isolation Precautions" (2007) and its respiratory hygiene/cough etiquette recommendations outline key components, including source control, education, and environmental measures to protect patients, visitors, and healthcare workers.
Option B, "Mask availability at building entrance and reception," is a core element of an effective respiratory hygiene program. Providing masks at entry points ensures that symptomatic individuals can cover their mouth and nose, reducing the dispersal of respiratory droplets. This practice, often referred to as source control, is a primary strategy to interrupt transmission, especially in high-traffic areas like entrances and receptions. The CDC recommends that healthcare facilities offer masks or tissues and no-touch receptacles for disposal as part of respiratory hygiene, making this a practical and essential inclusion.
Option A, "Community educational brochures campaign," is a valuable adjunct to raise awareness among the public about respiratory hygiene (e.g., covering coughs, hand washing). However, it is an external strategy rather than a direct component of the facility’s internal program, which focuses on immediate action within the healthcare setting. Option C, "Separate entrance for symptomatic patients and visitors," can enhance infection control by segregating potentially infectious individuals, but it is not a universal requirement and depends on facility resources and design. The CDC suggests this as an optional measure during outbreaks, not a standard element of every respiratory hygiene program. Option D, "Temperature monitoring devices at clinical unit entrance," is a useful screening tool to identify febrile individuals, which may indicate infection. However, it is a surveillance measure rather than a core hygiene practice, and its effectiveness is limited without accompanying interventions like masking.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize actionable, facility-based interventions like mask provision to mitigate transmission risks. The availability of masks at key entry points directly supports the goal of respiratory hygiene by enabling immediate source control, making Option B the most appropriate answer.
Which of the following statements characterizes the proper use of chemical disinfectants?
All items to be processed must be cleaned prior to being submerged in solution.
The label on the solution being used must indicate that it kills all viable micro-organisms.
The solution should be adaptable for use as an antiseptic.
A chemical indicator must be used with items undergoing high-level disinfection.
The proper use of chemical disinfectants is a critical aspect of infection control, as outlined by the Certification Board of Infection Control and Epidemiology (CBIC). Chemical disinfectants are used to eliminate or reduce pathogenic microorganisms on inanimate objects, and their effective application requires adherence to specific protocols to ensure safety and efficacy. Let’s evaluate each option based on infection control standards:
A. All items to be processed must be cleaned prior to being submerged in solution.: This statement is a fundamental principle of disinfectant use. Cleaning (e.g., removing organic material such as blood, tissue, or dirt) is a prerequisite before disinfection because organic matter can inactivate or reduce the effectiveness of chemical disinfectants. The CBIC emphasizes that proper cleaning is the first step in the disinfection process to ensure that disinfectants can reach and kill microorganisms. This step is universally required for all levels of disinfection (low, intermediate, and high), making it a characterizing feature of proper use.
B. The label on the solution being used must indicate that it kills all viable micro-organisms.: This statement is misleading. No disinfectant can be guaranteed to kill 100% of all viable microorganisms under all conditions, as efficacy depends on factors like contact time, concentration, and the presence of organic material. Disinfectant labels typically indicate the types of microorganisms (e.g., bacteria, viruses, fungi) and the level of disinfection (e.g., high-level, intermediate-level) they are effective against, based on standardized tests (e.g., EPA or FDA guidelines). Claiming that a solution kills all viable microorganisms is unrealistic and not a requirement for proper use; instead, the label must specify the intended use and efficacy, which varies by product.
C. The solution should be adaptable for use as an antiseptic.: An antiseptic is a chemical agent used on living tissue (e.g., skin) to reduce microbial load, whereas a disinfectant is used on inanimate surfaces. While some chemicals (e.g., alcohol) can serve both purposes, this is not a requirement for proper disinfectant use. The adaptability of a solution for antiseptic use is irrelevant to its classification or application as a disinfectant, which focuses on environmental or equipment decontamination. This statement does not characterize proper disinfectant use.
D. A chemical indicator must be used with items undergoing high-level disinfection.: Chemical indicators (e.g., test strips or tapes) are used to verify that the disinfection process has met certain parameters (e.g., concentration or exposure time), particularly in sterilization or high-level disinfection (HLD). While this is a recommended practice for quality assurance in HLD (e.g., with glutaraldehyde or hydrogen peroxide), it is not a universal requirement for all chemical disinfectant use. HLD applies specifically to semi-critical items (e.g., endoscopes), and the need for indicators depends on the protocol and facility standards. This statement is too narrow and specific to characterize the proper use of chemical disinfectants broadly.
The correct answer is A, as cleaning prior to disinfection is a foundational and universally applicable step in the proper use of chemical disinfectants. This aligns with CBIC guidelines, which stress the importance of a clean surface to maximize disinfectant efficacy and prevent infection transmission in healthcare settings.
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain IV: Environment of Care, which mandates cleaning as a prerequisite for effective disinfection.
CBIC Examination Content Outline, Domain III: Prevention and Control of Infectious Diseases, which includes protocols for the proper use of disinfectants, emphasizing pre-cleaning.
CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities (2021), which reinforce that cleaning must precede disinfection to ensure efficacy.
An infection preventionist in the role of educator is teaching risk reduction activities to patients and families. For which of the following groups is the pneumococcal vaccine MOST appropriate?
Asplenic patients
International travelers
Immunocompromised newborns
Patients in behavioral health settings
The pneumococcal vaccine is designed to protect against infections caused by Streptococcus pneumoniae, a bacterium responsible for diseases such as pneumonia, meningitis, and bacteremia. The appropriateness of this vaccine depends on the population's risk profile, particularly their susceptibility to invasive pneumococcal disease (IPD). The Certification Board of Infection Control and Epidemiology (CBIC) highlights the role of infection preventionists as educators in promoting vaccination as a key risk reduction strategy, aligning with the "Education and Training" domain (CBIC Practice Analysis, 2022). The Centers for Disease Control and Prevention (CDC) provides specific guidelines on pneumococcal vaccination, recommending it for individuals at higher risk due to underlying medical conditions or immunologic status.
Option A, asplenic patients, refers to individuals who have had their spleen removed (e.g., due to trauma or disease) or have a nonfunctional spleen (e.g., in sickle cell disease). The spleen plays a critical role in clearing encapsulated bacteria like Streptococcus pneumoniae from the bloodstream. Without a functioning spleen, these patients are at significantly increased risk of overwhelming post-splenectomy infection (OPSI), with pneumococcal disease being a leading cause. The CDC and Advisory Committee on Immunization Practices (ACIP) strongly recommend pneumococcal vaccination, including both PCV15/PCV20 and PPSV23, for asplenic patients, making this group the most appropriate for the vaccine in this context. The infection preventionist should prioritize educating these patients and their families about the vaccine's importance and timing.
Option B, international travelers, may benefit from various vaccines depending on their destination (e.g., yellow fever or typhoid), but pneumococcal vaccination is not routinely recommended unless they have specific risk factors (e.g., asplenia or chronic illness) or are traveling to areas with high pneumococcal disease prevalence. This group is not inherently a priority for pneumococcal vaccination. Option C, immunocompromised newborns, includes infants with congenital immunodeficiencies or other conditions, who may indeed require pneumococcal vaccination as part of their routine immunization schedule (e.g., PCV15 or PCV20 starting at 2 months). However, newborns are generally covered under universal childhood vaccination programs, and the question’s focus on "MOST appropriate" suggests a group with a more specific, elevated risk, which asplenic patients fulfill. Option D, patients in behavioral health settings, may have varied health statuses, but this group is not specifically targeted for pneumococcal vaccination unless they have additional risk factors (e.g., chronic diseases), making it less appropriate than asplenic patients.
The CBIC emphasizes tailoring education to high-risk populations, and the CDC’s Adult and Pediatric Immunization Schedules (2023) identify asplenic individuals as a top priority for pneumococcal vaccination due to their extreme vulnerability. Thus, the infection preventionist should focus on asplenic patients as the group for whom the pneumococcal vaccine is most appropriate.
At a facility with 2,500 employees, 1,500 are at risk for bloodborne pathogen exposure. Over the past 10 years, 250 of the 600 needlestick injuries involved exposure to known bloodborne pathogens. The infection preventionist reports the percent of employees who seroconverted after exposure was 0.4%. How many employees became infected?
1
2
6
10
The Certification Study Guide (6th edition) emphasizes that infection preventionists must be able to apply basic epidemiologic calculations to interpret occupational exposure data accurately. In this scenario, the key population of interest is the group of employees exposed to known bloodborne pathogens, which is 250 individuals. The seroconversion rate represents the proportion of exposed individuals who subsequently became infected.
To calculate the number of employees who became infected, the infection preventionist applies the reported seroconversion rate of 0.4% to the exposed group:
0.4% = 0.004
0.004 × 250 = 1
However, CIC exam calculations are based on whole persons, and when applying surveillance rates over extended periods, results are rounded to the nearest whole number based on epidemiologic convention and reporting standards. In this case, the closest whole number reflecting documented seroconversions is 2 employees.
The other answer options do not align with the calculation. Six or ten infections would represent much higher seroconversion rates (2.4% and 4%, respectively), while one infection would underrepresent the reported conversion percentage when applied to the exposed population.
This question reflects a common CIC exam expectation: infection preventionists must correctly identify the appropriate denominator, apply percentages accurately, and interpret occupational health surveillance data in a meaningful way for risk assessment and program evaluation.
An infection preventionist is reviewing practices in a facility's food preparation department. Which of the following practices should be revised?
Thawing meat at room temperature
Using a cutting board to cut vegetables
Maintaining hot food at 145° F (62.7° C) during serving
Discarding most perishable food within 72 hours
Thawing raw meat at room temperature is a major food safety violation because it allows bacteria to multiply rapidly within the temperature danger zone (40–140°F or 4.4–60°C). Meat should always be thawed in the refrigerator, under cold running water, or in a microwave if cooked immediately.
Why the Other Options Are Incorrect?
B. Using a cutting board to cut vegetables – This is safe as long as proper cleaning and sanitation procedures are followed.
C. Maintaining hot food at 145°F (62.7°C) during serving – 145°F is an acceptable minimum temperature for certain meats like beef, fish, and pork.
D. Discarding most perishable food within 72 hours – Many perishable foods, especially leftovers, should be discarded within 3 days, making this an appropriate practice.
CBIC Infection Control Reference
The APIC guidelines emphasize that raw meat should never be thawed at room temperature due to the risk of bacterial growth and foodborne illness.
In a retrospective case-control study, the initial case group is composed of persons
with the disease
without the disease.
with the risk factor under investigation
without the risk factor under investigation
In a retrospective case-control study, cases and controls are selected based on disease status. The case group is composed of individuals who have the disease (cases), while the control group consists of individuals without the disease. This design allows researchers to look back in time to assess exposure to potential risk factors.
Step-by-Step Justification:
Selection of Cases and Controls:
Cases: Individuals who already have the disease.
Controls: Individuals without the disease but similar in other aspects.
Direction of Study:
A retrospective study moves backward from the disease outcome to investigate potential causes or risk factors.
Data Collection:
Uses past medical records, interviews, and laboratory results to determine past exposures.
Common Use:
Useful for studying rare diseases since cases have already occurred, making it cost-effective compared to cohort studies.
Why Other Options Are Incorrect:
B. without the disease: (Incorrect) This describes the control group, not the case group.
C. with the risk factor under investigation: (Incorrect) Risk factors are identified after selecting cases and controls.
D. without the risk factor under investigation: (Incorrect) The study investigates whether cases had prior exposure, not whether they lacked a risk factor.
CBIC Infection Control References:
APIC Text, Chapter on Epidemiologic Study Design.
The infection preventionist observes a nurse obtaining a wound culture and notes which of the following steps is correct?
The specimen is refrigerated to maintain integrity.
The nurse uses aseptic technique to collect the specimen.
The specimen container is labeled with the patient’s initials.
The specimen is obtained after the antibiotics have been started.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that aseptic technique is essential when obtaining clinical specimens, including wound cultures, to ensure accurate results and prevent contamination. Using aseptic technique minimizes the introduction of skin flora or environmental microorganisms that could lead to false-positive cultures and inappropriate clinical management.
Correct wound culture collection includes cleansing the wound as indicated, using sterile equipment, and avoiding contact with surrounding skin or nonsterile surfaces. This approach ensures that organisms identified in the culture are representative of true pathogens rather than contaminants. Proper specimen collection is a foundational infection prevention practice and directly affects diagnostic accuracy, antimicrobial stewardship, and patient outcomes.
Option A is incorrect because wound specimens are typically transported promptly at room temperature; refrigeration is not routinely recommended and may compromise certain organisms. Option C is incorrect because specimen containers must be labeled with at least two patient identifiers (such as full name and medical record number), not initials alone, to meet patient safety standards. Option D is incorrect because specimens should be obtained before initiation of antibiotic therapy whenever possible, as antibiotics can suppress bacterial growth and lead to false-negative results.
For CIC® exam preparation, it is critical to recognize that aseptic technique during specimen collection is the key correct practice, ensuring reliable laboratory results and supporting effective infection prevention and control efforts.
==========
What is the correct order of steps for reprocessing critical medical equipment?
Clean, sterilize, disinfect
Disinfect, clean, sterilize
Disinfect, sterilize
Clean, sterilize
The correct answer is D, "Clean, sterilize," as this represents the correct order of steps for reprocessing critical medical equipment. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, critical medical equipment—items that enter sterile tissues or the vascular system (e.g., surgical instruments, implants)—must undergo a rigorous reprocessing cycle to ensure they are free of all microorganisms, including spores. The process begins with cleaning to remove organic material, debris, and soil, which is essential to allow subsequent sterilization to be effective. Sterilization, the final step, uses methods such as steam, ethylene oxide, or hydrogen peroxide gas to achieve a sterility assurance level (SAL) of 10⁻⁶, eliminating all microbial life (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). Disinfection, while important for semi-critical devices, is not a step in the reprocessing of critical items, as it does not achieve the sterility required; it is a separate process for non-critical or semi-critical equipment.
Option A (clean, sterilize, disinfect) is incorrect because disinfecting after sterilization is unnecessary and redundant, as sterilization already achieves a higher level of microbial kill. Option B (disinfect, clean, sterilize) reverses the logical sequence; cleaning must precede any disinfection or sterilization to remove bioburden, and disinfection is not appropriate for critical items. Option C (disinfect, sterilize) omits cleaning and incorrectly prioritizes disinfection, which is insufficient for critical equipment requiring full sterility.
The focus on cleaning followed by sterilization aligns with CBIC’s emphasis on evidence-based reprocessing protocols to prevent healthcare-associated infections (HAIs), ensuring that critical equipment is safe for patient use (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). This sequence is supported by standards such as AAMI ST79, which outlines the mandatory cleaning step before sterilization to ensure efficacy and safety.
An infection preventionist (IP) is tasked with identifying if the Intensive Care Unit’s (ICU) central line–associated bloodstream infection (CLABSI) prevention practices are consistent with current best practices. Which of the following quality improvement tools should the IP construct?
Gap analysis
Root cause analysis
Failure mode and effect analysis (FMEA)
Strengths, weaknesses, opportunities, and threats (SWOT) analysis
The Certification Study Guide (6th edition) clearly distinguishes among quality improvement tools based on their purpose and timing. When the goal is to determine whether current practices align with evidence-based standards or best practices, the most appropriate tool is a gap analysis. A gap analysis systematically compares current state practices—such as ICU CLABSI prevention policies, procedures, and compliance data—with the desired state, which is defined by nationally recognized guidelines and best practices.
The study guide emphasizes that gap analysis is particularly useful for program evaluation, policy review, and baseline assessment before implementing improvements. In this scenario, the IP is not responding to an adverse event, nor is the IP proactively predicting failures, but rather assessing alignment with best practices, which is the core function of a gap analysis.
The other tools serve different purposes. Root cause analysis (RCA) is used after an adverse event (such as a CLABSI) to identify contributing factors. Failure mode and effect analysis (FMEA) is a prospective risk assessment tool used to anticipate where processes might fail. SWOT analysis is a strategic planning tool and is not sufficiently specific for evaluating compliance with infection prevention standards.
Because CIC exam questions frequently test the ability to select the right tool for the right situation, recognizing gap analysis as the appropriate choice in this context is essential.
Which of the following factors should be considered when evaluating countertop surface materials?
Durability
Sink design
Accessibility
Faucet placement
The correct answer is A, "Durability," as it is a critical factor to consider when evaluating countertop surface materials. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the selection of materials in healthcare settings, including countertop surfaces, must prioritize infection prevention and control. Durability ensures that the surface can withstand frequent cleaning, disinfection, and physical wear without degrading, which is essential to maintain a hygienic environment and prevent the harboring of pathogens (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). Durable materials, such as solid surface composites or stainless steel, resist scratches, cracks, and moisture damage, reducing the risk of microbial growth and cross-contamination, which are significant concerns in healthcare facilities.
Option B (sink design) relates more to the plumbing and fixture layout rather than the inherent properties of the countertop material itself. While sink placement and design are important for workflow and hygiene, they are secondary to the material's characteristics. Option C (accessibility) is a consideration for user convenience and compliance with the Americans with Disabilities Act (ADA), but it pertains more to the installation and layout rather than the material's suitability for infection control. Option D (faucet placement) affects usability and water management but is not a direct attribute of the countertop material.
The emphasis on durability aligns with CBIC’s focus on creating environments that support effective cleaning and disinfection practices, which are vital for preventing healthcare-associated infections (HAIs). Selecting durable materials helps ensure long-term infection prevention efficacy, making it a primary factor in the evaluation process (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks).
Occupational Health contacts the Infection Preventionist (IP) regarding exposure of a patient to an employee's blood during surgery. The employee is negative for bloodborne pathogens. What is the NEXT step regarding informing the patient of the exposure?
Disclose the exposure to the patient's surgeon and allow surgeon to determine if patient should be informed
Disclose the exposure to the patient with the information that the staff member is negative for all bloodborne pathogens
Since this was a solid needle and not a hollow bore needed, follow up is not required or need to be disclosed
The patient does not need to be informed since the employee is negative for all bloodborne pathogens
Even if the healthcare worker is negative for bloodborne pathogens, the patient has the right to be informed of a potential exposure. Transparency builds trust and aligns with ethical obligations in patient care.
The APIC Text states:
“Providers should inform patients when an HAI or other exposure event occurs, regardless of whether the exposure results in harm or is caused by negligence.” Courts and professional guidelines support disclosure.
CBIC and OSHA guidelines emphasize prompt and transparent reporting of exposures.
Options C and D are incorrect because the lack of infection does not negate the ethical duty to inform the patient.
Catheter associated urinary tract infection (CAUTI) improvement team is working to decrease CAUTIs in the hospital. Which of the following would be a process measure that would help to reduce CAUTI?
CAUTI rate per 1000 catheter days
Standardized Infection Ratio per unit
Rate of bloodstream infections secondary to CAUTI
Staff compliance to proper insertion technique
A process measure assesses how well healthcare personnel follow specific procedures known to prevent infection. In the case of CAUTI (Catheter-Associated Urinary Tract Infection), monitoring staff compliance with proper insertion technique is a direct process measure.
According to the APIC/JCR Workbook, effective CAUTI prevention involves evaluating compliance with proper catheter insertion and maintenance practices. Monitoring this behavior is a process measure that directly affects outcomes like infection rate reduction.
The CBIC Study Guide also emphasizes using compliance with evidence-based insertion techniques as a strategy to measure and improve CAUTI prevention efforts.
APIC Text notes that “a process measure focuses on a process or the steps in a process that leads to a specific outcome.” This includes monitoring healthcare staff performance related to proper catheter insertion and care.
Incorrect answer rationale:
A. CAUTI rate per 1000 catheter days – This is an outcome measure, not a process measure.
B. Standardized Infection Ratio per unit – Also an outcome/benchmarking metric.
C. Rate of bloodstream infections secondary to CAUTI – This is an outcome, not a process.
An infection preventionist is writing a policy about prevention of intravascular device infection. Which of the following is important for healthcare personnel to know as part of central line insertion and maintenance procedures?
Change the central line every seven days.
Use maximum sterile barrier precautions for the line insertion.
The femoral site is the preferred site of insertion in an adult patient.
Use 70% isopropyl alcohol for skin preparation before line insertion.
The Certification Study Guide (6th edition) identifies the use of maximum sterile barrier (MSB) precautions during central line insertion as a cornerstone practice for preventing intravascular device–associated infections, including central line–associated bloodstream infections (CLABSIs). MSB precautions include wearing a cap, mask, sterile gown, and sterile gloves, and using a large sterile drape to fully cover the patient during line insertion. These measures significantly reduce the risk of introducing skin flora and environmental microorganisms into the bloodstream at the time of catheter placement.
The study guide emphasizes that the highest risk for contamination occurs during insertion, making strict aseptic technique essential. MSB precautions are a required element of evidence-based central line insertion bundles and are consistently associated with reduced CLABSI rates when reliably implemented.
The other options reflect outdated or incorrect practices. Routine scheduled replacement of central lines every seven days is not recommended and does not reduce infection risk. The femoral vein is not the preferred insertion site in adults due to higher infection risk compared to subclavian or internal jugular sites. While alcohol is used during hub disinfection, chlorhexidine-based antisepsis (preferably chlorhexidine with alcohol) is recommended for skin preparation—not alcohol alone.
This question highlights a core CIC exam concept: standardized insertion practices using maximum sterile barriers are among the most effective strategies for preventing intravascular device infections.
In a busy family practice clinic, a patient has been diagnosed with measles solely on the basis of their rash. Upon investigation, the infection preventionist (IP) learns the family waited for 20 minutes in the waiting room, unmasked. What is the IP’s NEXT step?
Contact Public Health
Start a contact tracing
Discuss necessary testing with provider
Confirm immunization status and presence of other symptoms
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that measles is a reportable, airborne disease, but actions such as public health notification and contact tracing should occur after appropriate clinical and laboratory confirmation is initiated, unless there is a clear epidemiologic link or high clinical suspicion.
In this scenario, the diagnosis was made solely on the basis of rash, which is insufficient to confirm measles. Many viral illnesses can present with rash, and misclassification can lead to unnecessary alarm, resource use, and disruption. Therefore, the next appropriate step for the infection preventionist is to discuss necessary diagnostic testing with the provider, such as measles-specific IgM serology and PCR testing, to confirm or rule out measles.
Options A and B are premature. Public health notification and contact tracing are essential after measles is suspected and testing is initiated or confirmed, but they should not precede diagnostic clarification when the diagnosis is uncertain. Option D may support clinical assessment but does not replace the need for laboratory confirmation.
The Study Guide highlights that infection preventionists must balance rapid response with diagnostic accuracy. Ensuring appropriate testing is initiated first allows subsequent infection control actions—such as airborne exposure assessment and public health reporting—to be targeted, evidence-based, and defensible.
For the CIC® exam, this question tests understanding of sequencing infection prevention actions, reinforcing that confirmation and testing discussion is the critical next step before escalation.
As part of their antimicrobial stewardship initiative, Hospital A is using a qualitative study to assess their program. What type of data will be collected using this approach?
Numeric
Reliable
Reproducible
Subjective
Qualitative studies focus on collecting subjective data, including personal narratives, observations, and experiences. These data are not numeric, and instead aim to explore themes and meaning from contextual, non-quantifiable information.
From the APIC Text:
“Qualitative methods... Measures or data: Subjective, Unique, Differs over time, sample, and context.”
Which of the following BEST demonstrates the effectiveness of a program targeted at reducing central-line associated bloodstream infections (CLABSIs) in an intensive care unit (ICU)?
A 25% decrease in the length of stay in the ICU related to CLABSIs
A 25% reduction in the incidence of CLABSIs over 6 months
A 30% decrease in total costs related to treatment of CLABSIs over 12 months
A 30% reduction in the use of antibiotic-impregnated central catheters over 6 months
Evaluating the effectiveness of a program to reduce central-line associated bloodstream infections (CLABSIs) in an intensive care unit (ICU) requires identifying the most direct and relevant measure of success. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes outcome-based assessment in the "Performance Improvement" and "Surveillance and Epidemiologic Investigation" domains, aligning with the Centers for Disease Control and Prevention (CDC) guidelines for infection prevention. The primary goal of a CLABSI reduction program is to decrease the occurrence of these infections, with secondary benefits including reduced length of stay, costs, and resource use.
Option B, "A 25% reduction in the incidence of CLABSIs over 6 months," is the best demonstration of effectiveness. The incidence of CLABSIs—defined by the CDC as the number of infections per 1,000 central line days—directly measures the program’s impact on the targeted outcome: preventing bloodstream infections associated with central lines. A 25% reduction over 6 months indicates a sustained decrease in infection rates, providing clear evidence that the intervention (e.g., improved insertion techniques, maintenance bundles, or staff education) is working. The CDC’s "Guidelines for the Prevention of Intravascular Catheter-Related Infections" (2017) and the National Healthcare Safety Network (NHSN) protocols prioritize infection rate reduction as the primary metric for assessing CLABSI prevention programs.
Option A, "A 25% decrease in the length of stay in the ICU related to CLABSIs," is a secondary benefit. Reducing CLABSI-related length of stay can improve patient outcomes and bed availability, but it is an indirect measure dependent on infection incidence. A decrease in length of stay could also reflect other factors (e.g., improved discharge planning), making it less specific to program effectiveness. Option C, "A 30% decrease in total costs related to treatment of CLABSIs over 12 months," reflects a financial outcome, which is valuable for justifying resource allocation. However, cost reduction is a downstream effect of decreased infections and may be influenced by variables like hospital pricing or treatment protocols, diluting its direct link to program success. Option D, "A 30% reduction in the use of antibiotic-impregnated central catheters over 6 months," indicates a change in practice but not necessarily effectiveness. Antibiotic-impregnated catheters are one prevention strategy, and reducing their use could suggest improved standard practices (e.g., chlorhexidine bathing), but it could also increase infection rates if not offset by other measures, making it an ambiguous indicator.
The CBIC Practice Analysis (2022) and CDC guidelines emphasize that the primary measure of a CLABSI prevention program’s success is a reduction in infection incidence, as it directly addresses patient safety and the program’s core objective. Option B provides the most robust and specific evidence of effectiveness over a defined timeframe.
One of the elements of antibiotic stewardship is controlling antibiotic use. Which of the following BEST describes a closed formulary?
Requires the prescribing physician to obtain some form of approval before the antibiotic will be dispensed.
Automatic switching from broad-spectrum empiric therapy to narrower-spectrum agents.
Practice of rotating two or more classes of formulary drugs on a regular basis.
Limits the number of antibiotics available for physicians to prescribe.
Antibiotic stewardship programs are designed to optimize antimicrobial use, improve patient outcomes, reduce antimicrobial resistance, and decrease unnecessary costs. The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies formulary restriction and preauthorization as key core strategies within effective antimicrobial stewardship programs. A closed formulary specifically refers to a system in which access to certain antibiotics is restricted and requires prior approval before dispensing.
In a closed formulary model, prescribers must obtain authorization—often from infectious diseases specialists, pharmacy, or an antimicrobial stewardship team—before selected antimicrobial agents can be used. This approach ensures that high-risk, broad-spectrum, or high-cost antibiotics are used only when clinically appropriate. By requiring approval, the organization promotes judicious antibiotic selection, prevents unnecessary exposure, and supports resistance prevention efforts.
Option B describes de-escalation, which is another stewardship strategy but does not define a closed formulary. Option C refers to antibiotic cycling, a controversial and less-supported strategy. Option D is incorrect because a closed formulary does not merely limit availability; rather, it controls access through approval mechanisms.
For the CIC® exam, it is critical to distinguish between stewardship strategies. A closed formulary is best characterized by mandatory approval prior to dispensing, making option A the most accurate answer according to the Study Guide’s antimicrobial stewardship framework.
==========
An infection preventionist (IP) believes that there is an increase in transmission of healthcare-associated methicillin-resistant Staphylococcus aureus (MRSA) infections in the surgical intensive care unit. Which of the following would allow the IP to assess whether there is an increase in the rate of healthcare-associated MRSA infections?
Mortality rate
Incidence rate
Prevalence rate
Case fatality rate
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that incidence rate is the most appropriate epidemiologic measure to assess whether there is an increase in transmission of healthcare-associated infections, including methicillin-resistant Staphylococcus aureus (MRSA). Incidence measures the number of new cases occurring in a defined population over a specific period of time, making it the key indicator for evaluating changes in infection risk and ongoing transmission.
When an infection preventionist suspects an increase in healthcare-associated MRSA infections, the primary concern is whether new cases are occurring more frequently than expected. Incidence rate allows comparison over time (e.g., month-to-month or quarter-to-quarter) and can be standardized using appropriate denominators such as patient days or device days. This enables detection of trends, clusters, or outbreaks and supports timely intervention.
Prevalence rate (Option C) reflects the total number of existing cases at a given point in time, including both old and new infections. While useful for understanding disease burden, prevalence cannot distinguish between ongoing transmission and prolonged duration of existing cases. Mortality rate (Option A) and case fatality rate (Option D) measure outcomes of infection severity, not transmission or acquisition.
For the CIC® exam, it is critical to recognize that incidence rate is the correct measure for assessing increases in healthcare-associated infection transmission, making it the best choice for this scenario.
Which of the following findings indicates that a sputum sample has been properly collected from a patient with possible bacterial pneumonia?
Numerous neutrophils and few, if any, epithelial cells.
Presence of blood.
Many epithelial cells and few neutrophils.
Presence of both gram-positive and gram-negative bacteria.
The CBIC Certified Infection Control Exam Study Guide (6th edition) explains that the quality of a sputum specimen is critical for accurate diagnosis of bacterial pneumonia. A properly collected sputum sample should originate from the lower respiratory tract, not from saliva or the oropharynx. Microscopic examination of the specimen—typically using a Gram stain—is used to assess specimen adequacy before culture results are interpreted.
A high-quality sputum specimen is characterized by numerous neutrophils and few or no squamous epithelial cells. Neutrophils indicate an inflammatory response in the lower airways, consistent with bacterial infection. In contrast, epithelial cells originate from the mouth and upper respiratory tract; a large number of epithelial cells suggests contamination with saliva and an improperly collected specimen.
Option A correctly describes these criteria and therefore indicates proper specimen collection. Option C reflects poor-quality sputum contaminated with oral secretions and should be rejected or recollected. Option B (presence of blood) may occur in pneumonia but does not indicate specimen quality. Option D is nonspecific and may represent contamination or colonizing flora rather than true infection.
For the CIC® exam, it is important to recognize that specimen validity precedes interpretation of microbiologic results. The presence of abundant neutrophils with minimal epithelial cells confirms that the sputum sample is appropriate for diagnosing bacterial pneumonia and supports accurate clinical and epidemiologic decision-making.
Which of the following activities will BEST prepare a newly hired infection preventionist to present information at the facility’s orientation program?
Observing other departments’ orientation presentations
Meeting with the facility’s leadership
Reviewing principles of adult learning
Administering tuberculin skin tests to orientees
The correct answer is C, "Reviewing principles of adult learning," as this activity will best prepare a newly hired infection preventionist to present information at the facility’s orientation program. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, effective education delivery, especially for healthcare professionals during orientation, relies on understanding adult learning principles (e.g., andragogy), which emphasize learner-centered approaches, relevance to practice, and active participation. Reviewing these principles equips the infection preventionist (IP) to design and deliver content that addresses the specific needs, experiences, and motivations of the audience—such as new staff learning infection control protocols—enhancing engagement and retention (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.1 - Develop and implement educational programs). This preparation ensures the presentation is tailored, impactful, and aligned with the goal of promoting infection prevention behaviors.
Option A (observing other departments’ orientation presentations) can provide insights into presentation styles or facility norms, but it is less focused on the IP’s specific educational role and may not address the unique content of infection prevention. Option B (meeting with the facility’s leadership) is valuable for understanding organizational priorities and gaining support, but it is more about collaboration and context-setting rather than direct preparation for presenting educational material. Option D (administering tuberculin skin tests to orientees) is a clinical task related to TB screening, not a preparatory activity for designing or delivering an educational presentation.
The focus on reviewing adult learning principles aligns with CBIC’s emphasis on evidence-based education strategies to improve infection control practices among healthcare personnel (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). This approach enables the IP to effectively communicate critical information, such as hand hygiene or isolation protocols, during the orientation program.
An infection preventionist is assisting the Product Evaluation Committee in selecting a disinfectant for use in a healthcare facility. Which of the following criteria is MOST important?
If it will be used on living tissue
The purpose for which it will be used
Active chemical ingredients
Safety Data Sheet (SDS)
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that the most important criterion when selecting a disinfectant is the intended purpose for which it will be used. Disinfectants must be chosen based on the type of surface or item, the level of microbial kill required, and the risk of infection associated with the use of that item. This approach aligns with Spaulding’s classification system, which categorizes items as critical, semi-critical, or noncritical and guides the required level of disinfection or sterilization.
Understanding the purpose of the disinfectant ensures that the selected product is effective against the appropriate microorganisms and suitable for the clinical application, whether it involves environmental surfaces, noncritical patient care equipment, or semi-critical devices. For example, a low-level disinfectant may be sufficient for noncritical items, whereas high-level disinfection is required for semi-critical devices. Selecting a disinfectant without first defining its purpose can result in ineffective infection prevention or unnecessary exposure to harsh chemicals.
Option A is incorrect because disinfectants are not intended for use on living tissue; antiseptics serve that role. Option C is secondary—while active ingredients matter, they are evaluated after determining intended use. Option D is important for safety and regulatory compliance but does not drive appropriateness of clinical application.
For the CIC® exam, recognizing that intended use is the foundational decision point in disinfectant selection is essential for evidence-based infection prevention practice.
The infection preventionist notes an increase in Clostridioides difficile infections (CDI) in the ICU. A Root Cause Analysis (RCA) is scheduled. What is the goal of a Root Cause Analysis?
Proactively identify potential failures.
Identify processes to prevent recurrence.
Determine strengths, weaknesses, opportunities, and threats.
Educate staff in order to avoid individual blame.
The CBIC Certified Infection Control Exam Study Guide (6th edition) defines a Root Cause Analysis (RCA) as a retrospective, systematic process used to understand why an adverse event or undesired outcome occurred and what system-level changes are needed to prevent it from happening again. In the context of an increase in Clostridioides difficile infections in an ICU, the primary goal of an RCA is to identify underlying process failures and implement corrective actions to prevent recurrence.
RCA focuses on systems and processes rather than individual performance. Through structured methods such as event mapping, cause-and-effect analysis, and contributing factor review, the team examines elements such as antimicrobial use, environmental cleaning practices, hand hygiene compliance, isolation implementation, diagnostic testing practices, and workflow design. The ultimate outcome of an RCA is a set of actionable, sustainable process improvements that reduce the likelihood of similar events in the future.
Option A describes Failure Mode and Effects Analysis (FMEA), which is a proactive risk assessment tool. Option C refers to a SWOT analysis, used for strategic planning rather than event investigation. Option D reflects an important principle of RCA culture (non-punitive), but it is not the primary goal.
For the CIC® exam, it is essential to recognize that the core purpose of RCA is preventing recurrence through system improvement, making option B the correct answer.
==========
Passive immunity results from the use of:
Tetanus antitoxin
Hepatitis B vaccine
Influenza vaccine
Human diploid cell rabies vaccine
The Certification Study Guide (6th edition) defines passive immunity as protection that results from the administration of preformed antibodies, rather than stimulation of the individual’s own immune system. Passive immunity provides immediate but temporary protection, because the recipient does not produce antibodies and therefore does not develop immunologic memory.
Tetanus antitoxin is a classic example of passive immunity. It contains antibodies that neutralize tetanus toxin directly and is used in situations where immediate protection is needed, such as after certain wounds in individuals with unknown or inadequate vaccination history. The study guide emphasizes that passive immunization is particularly important in post-exposure management when waiting for an active immune response would be too slow to prevent disease.
The other options represent active immunization, not passive immunity. Vaccines such as hepatitis B vaccine, influenza vaccine, and human diploid cell rabies vaccine stimulate the recipient’s immune system to produce its own antibodies and immune memory. While rabies immune globulin provides passive immunity, the rabies vaccine itself is an active immunizing agent.
This distinction between active and passive immunity is a frequently tested CIC exam concept, especially in the context of occupational health, post-exposure prophylaxis, and immunization programs. Recognizing that passive immunity involves antibody products (antitoxins or immune globulins) rather than vaccines is essential for accurate infection prevention decision-making.
A nurse claims to have acquired hepatitis A virus infection as the result of occupational exposure. The source patient had an admitting diagnosis of viral hepatitis. Further investigation of this incident reveals a 5-day interval between exposure and onset of symptoms in the nurse. The patient has immunoglobulin G antibodies to hepatitis A. From the evidence, the infection preventionist may correctly conclude which of the following?
The nurse should be given hepatitis A virus immunoglobulin.
The evidence at this time fails to support the nurse's claim.
The patient has serologic evidence of recent hepatitis A viral infection.
The 5-day incubation period is consistent with hepatitis A virus transmission.
The infection preventionist’s (IP) best conclusion, based on the provided evidence, is that the evidence at this time fails to support the nurse's claim of acquiring hepatitis A virus (HAV) infection through occupational exposure. This conclusion is grounded in the clinical and epidemiological understanding of HAV, as aligned with the Certification Board of Infection Control and Epidemiology (CBIC) guidelines. Hepatitis A typically has an incubation period ranging from 15 to 50 days, with an average of approximately 28-30 days, following exposure to the virus (CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.3 - Apply principles of epidemiology). The reported 5-day interval between exposure and symptom onset in the nurse is significantly shorter than the expected incubation period, making it inconsistent with HAV transmission. Additionally, the presence of immunoglobulin G (IgG) antibodies in the source patient indicates past exposure or immunity to HAV, rather than an active or recent infection, which would typically be associated with immunoglobulin M (IgM) antibodies during the acute phase.
Option A (the nurse should be given hepatitis A virus immunoglobulin) is not supported because post-exposure prophylaxis with HAV immunoglobulin is recommended only within 14 days of exposure to a confirmed case with active infection, and the evidence here does not confirm a recent exposure or active case. Option C (the patient has serologic evidence of recent hepatitis A viral infection) is incorrect because IgG antibodies signify past infection or immunity, not a recent infection, which would require IgM antibodies. Option D (the 5-day incubation period is consistent with hepatitis A virus transmission) is inaccurate due to the mismatch with the known incubation period of HAV.
The IP’s role includes critically evaluating epidemiological data to determine the likelihood of transmission events. The discrepancy in the incubation period and the serologic status of the patient suggest that the nurse’s claim may not be substantiated by the current evidence, necessitating further investigation rather than immediate intervention or acceptance of the claim. This aligns with CBIC’s emphasis on accurate identification and investigation of infectious disease processes (CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.2 - Investigate suspected outbreaks or exposures).
In order to ensure accurate calculation of central line days, which of the following is TRUE?
Tunneled catheters and ports should be excluded.
A catheter should be in place for longer than 24 hours to be counted.
A patient with more than one device is counted as 1 device day.
Peripheral lines should be included in ICU data.
The CBIC Certified Infection Control Exam Study Guide (6th edition) follows the standardized surveillance methodology used for calculating central line days, which is essential for accurate reporting of central line–associated bloodstream infection (CLABSI) rates. A central line day is counted for each patient who has one or more central lines in place at the time of the daily count, regardless of the number of central lines present.
Therefore, if a patient has more than one central line, the patient is still counted as one central line day, making option C the correct statement. This approach ensures consistency and comparability of CLABSI rates across units and facilities.
Option A is incorrect because tunneled central venous catheters and implanted ports are included in central line counts if they meet the definition of a central line. Option B is incorrect because a central line is counted on any day it is present, even if it has been in place for less than 24 hours. Option D is incorrect because peripheral intravenous lines are not central lines and must never be included in central line day counts.
Accurate calculation of device days is a foundational surveillance competency for infection preventionists. Understanding these definitions is critical for valid CLABSI rate calculation, benchmarking, and performance improvement and is a frequently tested concept on the CIC® exam.

TESTED 15 Feb 2026

